Background
Immune thrombocytopenia (ITP) results from autoimmune antibody-mediated destruction of platelets. Bruising and bleeding are hallmarks of ITP, but thromboembolic events (TEEs) are also observed in ITP patients, even thrombocytopenic ones. In population-based cohort studies, chronic ITP patients had two-fold higher risk of a venous TEE compared to the general population.1 The risk of venous or arterial TEE is further elevated in splenectomized ITP patients and those on thrombopoietin receptor agonists (TPO-RAs). Other risk factors include: age > 60 years, prolonged corticosteroid treatment, diabetes, hypertension, coronary disease, history of TEEs, presence of anti-phospholipid antibodies, chronic kidney disease, and male sex.
Fostamatinib, an inhibitor of spleen tyrosine kinase (SYK), is approved for treatment of thrombocytopenia in adults with ITP. Extensive preclinical data suggest that inhibition of SYK signaling may also have an anti-thrombotic effect. In a model of acute stroke, mice with platelet-specific SYK deficiency were protected from aortic thrombosis and also had diminished size of brain infarction with only marginal effect on hemostasis.2 These results were reproduced by a SYK inhibitor in this model.2 We assessed rates of thrombosis among ITP patients treated with fostamatinib compared to those reported with TPO-RAs.
Methods
We reviewed the incidence of TEEs during fostamatinib clinical trials in ITP, including 2, randomized, double-blind, placebo-controlled, phase 3, multicenter clinical trials and an open-label extension study. The incidence of TEEs with TPO-RAs during published phase 3 clinical trials and post-phase 3 extension studies was also reviewed.
Results
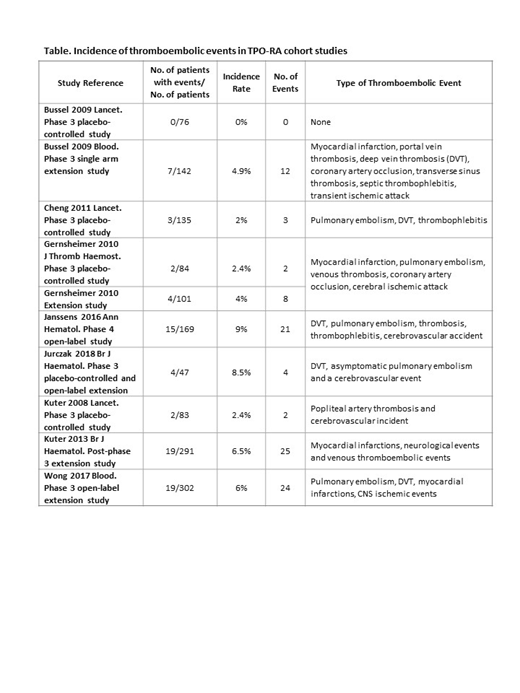
The study population comprised 145 ITP patients who received fostamatinib and included 37 (26%) patients >65 years, 51 (35%) splenectomized patients, and 58 (40%) male patients. The 3 fostamatinib clinical trials represent 163 patient-years of fostamatinib exposure. The only reported TEE was a transient ischemic attack (0.7%), which resolved spontaneously in a patient with pre-existing atherosclerosis. The rate of TEEs reported in ITP patients receiving TPO-RAs in multiple studies ranged from 0 to 9% (Table).
Discussion
The rate of TEEs observed in ITP patients receiving fostamatinib was very low (< 1%) in comparison with the rates of TEEs reported in ITP patients receiving a TPO-RA (up to 9%). ITP patients have higher levels of prothrombin fragments 1 + 2, D-dimer, PAI-1 and soluble P-selectin, which contribute to a procoagulant profile. TPO-RA treatment may cause an increase in PAI-1, P-selectin, and increase microparticles and platelet apoptosis, all of which predispose to onset of TEEs.3,4
What explains the apparent paradox of fostamatinib? SYK plays a key role in signaling of the ITAM receptors GPVI and CLEC-2 in platelets. Both GPVI and CLEC-2 play an important role in thrombosis but are dispensable for normal hemostasis. Consequently, SYK inhibition likely reduces the incidence of TEEs by abrogating the GPVI and CLEC-2 pathways in platelets. R406, the active metabolite of fostamatinib, diminished GPVI and CLEC-2 mediated platelet aggregation with marginal effect on normal hemostasis in vivo.2,5 These mechanisms potentially explain why ITP patients treated with fostamatinib are protected from developing TEEs despite often substantial platelet increases.
SYK also plays a key role in Fcγ receptor dependent phagocytosis of auto-antibody coated platelets, the primary disease mechanism in ITP. Thus, SYK inhibition in ITP may simultaneously decrease platelet destruction (increasing platelet count) and decrease the incidence of TEEs.
Suumary
Current clinical trial experience with fostamatinib in ITP patients demonstrates much lower rates of thrombosis than would be expected in ITP patients compared to other agents. Future work will prospectively evaluate this and confirm in vivo downregulation of platelet activation via inhibition of SYK signaling in platelets.
1. Rodeghiero F. Am J Hematol, 2016. 91: 39-45.
2. van Eeuwijk JM, et al. Arterioscler Thromb Vasc Biol, 2016. 36: 1247-53.
3. Garabet, L., et al. Platelets, 2019. 30: 206-12.
4. Justo Sanz, R., et al. Thromb Haemost, 2019. 119: 645-59.
5. Spalton, J.C., et al. J Thromb Haemost, 2009. 7: 1192-9.
Altomare:Novartis: Consultancy; Amgen: Consultancy; Rigel: Consultancy; Incyte: Consultancy, Speakers Bureau. Markovtsov:Rigel: Employment, Equity Ownership. Todd:Rigel: Employment, Equity Ownership. Weerasinghe:Rigel: Employment. Numerof:Rigel: Employment, Equity Ownership. Tong:Rigel: Employment, Equity Ownership. Masuda:Rigel: Consultancy, Employment, Equity Ownership. Bussel:Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; 3S Bio: Speakers Bureau; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; UCB: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; argenx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; RallyBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kezar Life Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Physician Education Resource: Speakers Bureau; Tranquil: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dova Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Momenta Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Fostamatinib is a tyrosine kinase (SYK) inhibitor for the treatment of adult patients with chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment. The use of fostamatinib in other diseases is off-label.
Author notes
Asterisk with author names denotes non-ASH members.