Introduction: Cancer patients have approximately 4 times higher risk of developing venous thromboembolism (VTE) compared to the general population. High tendency of bleeding from anticoagulant use in this population makes the treatment of cancer-associated thromboembolism (CAT) very challenging. Low molecular weight heparin (LMWH) is still considered as standard treatment for CAT. Direct oral anticoagulants (DOAC) have emerged as a potential alternative for LMWH due to the ease of administration and predictable pharmacokinetics, but data on DOACs in CAT is limited. Few randomized controlled trials (RCT) published recently have compared the efficacy and safety of DOACs with LMWH in the treatment of CAT. Hence, we conducted an updated meta-analysis of RCTs to determine the relative risk of recurrent VTE and bleeding complications associated with DOACs compared to LMWH in the treatment of thromboembolism in patients with cancer, and to evaluate if the risk estimates have changed since prior report (Li et al.).
Methods: We performed a systemic search using Embase, Medline, and the meeting abstracts with appropriate keywords through 06/30/19, to find all RCTs comparing a DOAC with LMWH in treatment of patients with CAT. The search strategy, study selection, data extraction and analysis were performed in accordance with the Preferred Reporting Items for Meta-Analyses (PRISMA) guidelines. We pooled the point estimates in form of risk ratios (RR) with respective 95% confidence intervals (CI), using the random effects model (Mantel-Haenszel method) of Der Simonian and Laird. Heterogeneity of effect size across studies was quantified using I2 statistic and Cochran's Q. Publication bias was assessed by the Egger's regression test. All the statistical analyses were performed with the RevMan 5.3 software. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014.
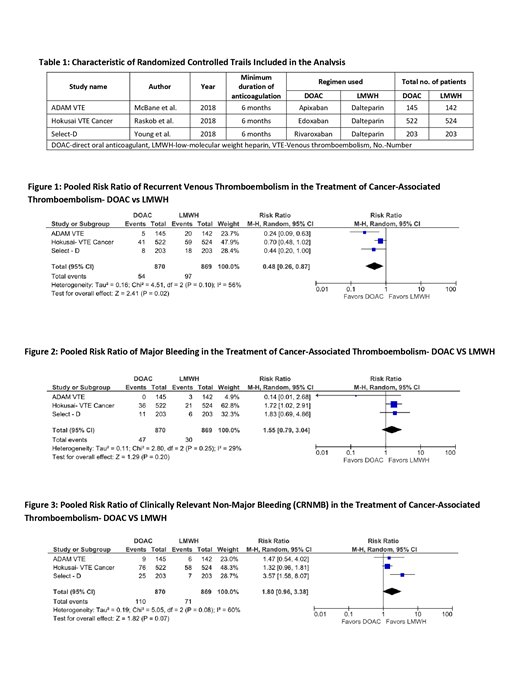
Results: Overall a total of 1,739 patients with CAT (870 in the DOAC arms and 869 in LMWH arms) from three RCTs were included in the final analysis. Characteristics of studies included in the analysis are summarized in table 1. Different DOACs (Select-D: rivaroxaban, Hokusai VTE cancer: edoxaban and ADAM VTE: apixaban) were used to compare with dalteparin in included trials. Duration of anticoagulation was 6 to 12 months in these studies. Use of DOAC was associated with a significantly lower risk of recurrent VTE in comparison with LMWH [pooled RR 0.48, 95%CI: 0.26-0.87, p = 0.02, I2 = 56%, figure 1]. In addition, there was no statistically significant increase in the risk of major bleeding in patients on the DOAC arms, as compared to those on LMWH arms [ pooled RR 1.55 ,95%CI: 0.79-3.04, p = 0.20, I2 = 29%, figure 2]. Criteria for major bleeding in the studies were defined by the International Society on Thrombosis and Hemostasis. The pooled RR for clinically relevant non major bleeding (CRNMB) was 1.80 [95%CI: 0.96-3.38, p = 0.07, I2 = 60%, figure 3], thus suggesting no significant difference in risk of CRNMB between DOAC and LMWH groups. Moderate heterogeneity was noted across trials. We found no publication bias among studies included in the analysis.
Conclusion: In our meta-analysis, use of DOACs for the treatment of CAT was associated with a significantly decreased risk of recurrent VTE compared to LMWH. There was no significant difference in the incidence of major or non-major bleeding events between DOAC and LMWH groups. These study results provide additional evidence for potential use of DOAC as a safe and effective alternative to LMWH for the treatment of thromboembolism in patients with cancer.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.