Background. Acute myeloid leukemia (AML) is the most common acute leukemia in adults. The median age of diagnosis is 67 years old, and it has unfavorable outcomes in older patients. Approximately one-third of patients are diagnosed after the age of 75. Thus, as the population continues to increase in age, the incidence of AML will continue to expand (NCCN guidelines: AML. Version 3.2019). The long term disease free survival (DFS) rates for patients > 60 years of age is 5-15% whereas younger patients < 60 years of age have a better DFS rate of up to 40% (Dohner H, et al. N Engl J Med. 2015). Recent advancements have been made in patients with AML, including the approval of daunorubicin and cytarabine liposomal (Vyxeos®) for the treatment of adults with 2 poor risk types of AML: newly diagnosed therapy related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC). Given the financial constraints of this new medication, our objective was to determine the safety and efficacy of daunorubicin and cytarabine liposomal in our adult patients with t-AML and AML-MRC at a single academic medical center.
Methods. This was a single center, retrospective, chart review at Wake Forest Baptist Health (WFBH) Comprehensive Cancer Center from August 1, 2017 to November 1, 2018. Patients were selected via report generation if they had received at least one dose of daunorubicin and cytarabine liposomal during the study period. The initial induction dose of daunorubicin and cytarabine liposomal was 44 mg/m2 of daunorubicin and 100 mg/m2 cytarabine administered on days 1, 3, and 5 for up to 2 cycles to achieve remission. If a second induction was necessary, the same induction doses were given on days 1 and 3 only. The consolidation dose was 29 mg/m2 of daunorubicin and 65 mg/m2 of cytarabine on days 1 and 3 for up to 2 cycles. The primary endpoint was overall survival (OS). Secondary endpoints included event free survival (EFS), 30-day and 60-day mortality, complete remission (CR) and morphologic complete remission with incomplete blood count recovery (CRi), adverse drug reactions, and financial impact to the health system. Descriptive statistics were utilized for demographic data. Time to event data was analyzed using the Kaplan-Meier method. SPSS IBM and Microsoft Excel Software were utilized for data analysis.
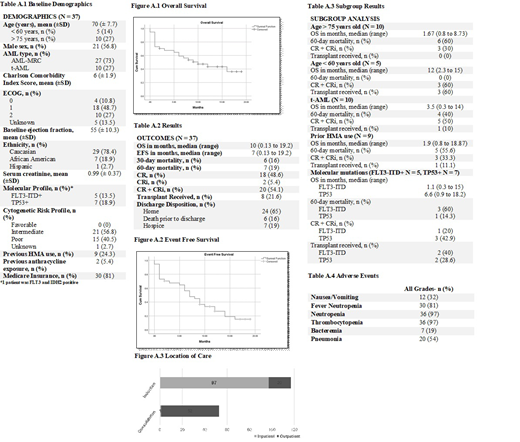
Results. A total of 37 AML patients were identified as receiving daunorubicin and cytarabine liposomal from August 2017 to November 2018. Of those 37 patients, 27 had AML-MRC and 10 had t-AML. The average patient was a 70 year old Caucasian male with an ECOG performance status of 1 and a Charlson Comorbidity Index of 6 (Table 1). The median OS was 10 months and EFS was 7 months. The 30-day mortality rate was 16% and 60-day mortality rate was 19%. Eighteen patients (49%) achieved a CR and 2 patients (5%) achieved a CRi. A subgroup analysis was conducted for prior hypomethylating agent (HMA) use, age > 75 years old, < 60 years old, molecular mutations including FLT3-ITD and TP53 mutations, and t-AML. Poorer outcomes were noted in patients > age 75, prior HMA use, and the t-AML subgroups. Table 3 highlights the OS, 60-day mortality rate, transplant received and CR+CRi for each subgroup. The median time to platelet and absolute neutrophil count (ANC) recovery was 32 and 33 days, respectively. Eight patients (21.6%) proceeded to transplant post administration of daunorubicin and cytarabine liposomal. All patients experienced at least one adverse event with hematologic being the most commonly observed toxicity (Table 4). Most patients received induction therapy with daunorubicin and cytarabine liposomal in the inpatient setting whereas consolidation was predominantly administered in an outpatient encounter.
Conclusions. Daunorubicin and cytarabine liposomal was considered an effective treatment option for patients with t-AML and AML-MRC with a CR+CRi rate of 54%. Younger patients (< 60 years old) exhibited the greatest benefit with an OS of 12 months and 60 day mortality rate of 0%. However, poorer outcomes were demonstrated in elderly patients (> 75 years old), patients with FLT3-ITD positive mutations, and patients with previous HMA use, with an OS less than 2 months in each subgroup and mortality rates ranging up to 60%. Thus, additional studies are necessary to determine the role of daunorubicin and cytarabine liposomal in these higher risk patient subgroups > age 75, FLT3-ITD positive patients, and patients with previous HMA use.
Manuel:Novartis: Speakers Bureau; Jazz Pharmaceuticals: Speakers Bureau. Pardee:Rafael Pharmaceuticals: Consultancy, Research Funding; Karyopharm: Research Funding; Spherix Intellectual Property: Research Funding; Pharmacyclics/Janssen: Speakers Bureau; Celgene: Speakers Bureau; Amgen: Speakers Bureau; CBM Bipharma: Membership on an entity's Board of Directors or advisory committees. Powell:Janssen: Research Funding; Rafael Pharmaceuticals: Consultancy, Research Funding; Novartis: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.