Introduction
In 2008, the World Health Organization defined a new classification of myeloid and lymphoid neoplasms with eosinophilia that result from gene rearrangements of PDGFRA, PDGFRB, and FGFR1. While rearrangements involving PDGFRA and PDGFRB generally respond well to imatinib, those associated with FGFR1 are typically aggressive and require treatment with allogeneic hematopoietic stem cell transplantation (SCT). Here we present the case of a patient with a previously unreported fusion of PCM1-FGFR1. The patient was treated with an Oral, potent, selective, and irreversible small-molecule inhibitor of FGFR 1- 4 (futibatinib (TAS-120)) under an expanded access program, resulting in the first reported instance of complete hematologic and cytogenetic remission using futibatinib in an FGFR-driven myeloid neoplasm.
Results
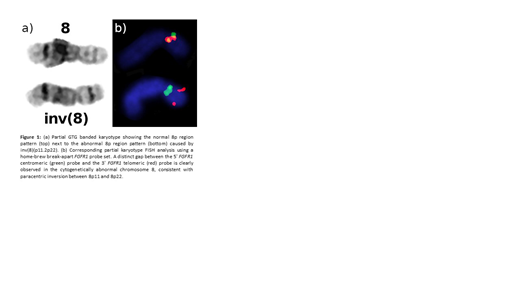
A 55-year-old male presented with dyspnea and fatigue and was found to have peripheral eosinophilia (3,660/microliter) and thrombocytopenia (46,000/microliter). Diagnostic bone marrow biopsy was notable for a hypercellular (cellularity >95%), erythroid dominant marrow with increased eosinophilic forms and increased pronormoblasts. Break-apart fluorescence in situ hybridization (FISH) studies revealed an FGFR1 gene rearrangement in 11.3% of nuclei (normal < 5.7%). The nature of the rearrangement was shown to be a paracentric inversion of chromosome 8p based on the distinct gap between the 5'FGFR1 and 3'FGFR1 probes in metaphase FISH (Figure 1). A validated, targeted next generation sequencing assay for fusion transcript detection (heme fusion assay) revealed a previously unreported PCM1-FGFR1 fusion transcript (40 unique fusion reads), with an in-frame fusion of PCM1 (exons 1-36) to FGFR1 (exons 11-18). No additional clonal markers were identified.
The patient was not considered an SCT candidate due to medical comorbidities and was enrolled on a single-patient protocol expanded access program for futibatinib. He was initially treated with prednisone for control of his eosinophilia, and then started on oral therapy with futibatinib (20 mg daily). Within 1 month of initiation of futibatinib, prednisone was tapered without recurrence of eosinophilia and with improvement in platelet count (169,000/microliter). After 6 months, repeat bone marrow biopsy showed a moderately hypocellular marrow with maturing trilineage hematopoiesis. Additionally, the paracentric inversion of chromosome 8p was no longer observed in metaphase FISH, consistent with cytogenetic remission. Furthermore, the PCM1-FGFR1 fusion transcript was no longer detectable by heme fusion assay.
The patient has experienced grade 2 skin rash requiring brief dose interruption (7 days) followed by dose reduction to 16 mg daily, on which he remains. He has also experienced grade 2 hyperphosphatemia, a known side effect of futibatinib, which is adequately controlled with sevelamer. The patient continues on futibatinib, with ongoing evidence of hematologic and cytogenetic remission after 11 months of therapy.
Conclusions
To our knowledge, this case represents the first report of a PCM1-FGFR1 fusion driving a myeloid neoplasm with eosinophilia. Treatment with futibatinib has resulted in hematologic and cytogenetic remission, with treatment successfully ongoing after 11 months. Our findings support further exploration of FGFR inhibitors as a therapeutic strategy for myeloid/lymphoid neoplasms driven by FGFR1 rearrangement, particularly in individuals who are not candidates for SCT. A phase 2 study of futibatinib in patients with FGFR1 driven myeloid/lymphoid neoplasms is planned.
Brunner:Astra Zeneca: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Forty Seven Inc: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding. Chen:Magenta: Consultancy; Takeda: Consultancy; Kiadis: Consultancy; Incyte: Consultancy; Abbvie: Consultancy. Fathi:Amphivena, Kite, Jazz, NewLink Genetics,: Honoraria; Agios, Astellas, Celgene, Daiichi Sankyo, Novartis, Takeda, Amphivena, Kite, Forty Seven,Trovagene, NewLink genetics, Jazz, Abbvie, and PTC Therapeutics: Consultancy. Narayan:Genentech: Other: Equity ownership (spouse); Merck: Other: Equity ownership (spouse); Takeda: Other: Employment (spouse). Benhadji:Taiho Oncology: Employment. Hobbs:Incyte: Consultancy, Research Funding; Merck: Research Funding; Jazz pharmaceuticals: Consultancy; Celgene: Consultancy; Bayer: Research Funding; Agios: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.