Introduction
As has been described by Dr. Viswabandya et al, the combination of anti-thymoglobulin (ATG), post-transplant cyclophosphamide (PTCy) and cyclosporine (CsA) provides an effective control of graft-versus host disease (GVHD) in peripheral blood (PB) allo-HSCT.
We aim to report our experience in reduced intensity conditioning (RIC) allo-HSCT using a novel GVHD prophylaxis composed by a very low dose of ATG, PTCy and CsA for GVHD prophylaxis.
Patients and methods
Between May 2018 and April 2019, 106 adult patients underwent RIC allo-HSCT with the present GVHD prophylaxis. All these recipients were included in the study.
RIC regimen was composed by fludarabine, busulfan, and 200cGy of total body irradiation. For GVHD prophylaxis all recipients received a total dose of 2mg/kg of rabbit-ATG on day -3 (0.5mg/kg) and -2 (1.5mg/kg), PTCy 50mg/m2/day on day +3 and +4, and CsA since day +5.
Data was collected retrospectively and updated on July 2019. The median follow-up was 7.6 months (range: 0.4-14.6) and survival percentages were calculated at 6 months. The cum.Inc of GVHD was assessed accounting relapse and death as competing events.
Results
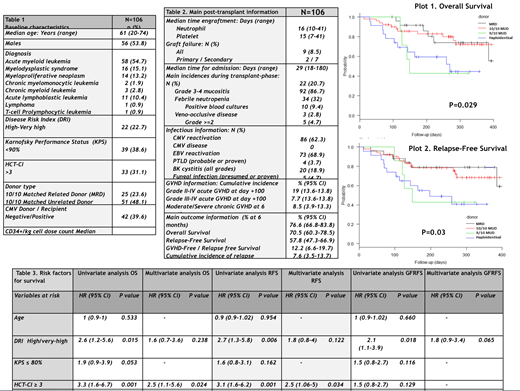
The main baseline and post-transplant information is summarized in the Table 1 and 2.
The cum.Inc of grade II-IV and III-IV acute GVHD at day +100 was 19% and 7.7%. The cum.Inc of grade II- IV was not significantly affected by donor type (P=0.787). However, the cum.Inc of grade III-IV was significantly higher in those recipients who received haploidentical or 9/10 matched unrelated donor (MUD) grafts (p=0.048).
Rates of CMV and EBV reactivation were respectively 62.3% and 68.9%. No patients were diagnosed with CMV disease. Biopsy proven post-transplant lymphoproliferative disorder was diagnosed in only 1 recipient and successfully resolved after rituximab.
Thirty-two (30.2%) recipients died and 16 (15.1%) relapsed during the follow-up. Main causes of death were relapse 13 (12.3%) and infection 6 (5.7%). The main outcome information is reported in the Table 2 and Plot 1 and 2.
Those recipients who received grafts from matched related and unrelated donors had a significant better non-relapse mortality (NRM) (P=0.001), overall survival (OS) (P=0.02), relapse-free survival (RFS) (P=0.03) and GVHD-free/RFS (P=0.006).
Table 3 shows the impact of risk factors in transplant outcomes. Those recipients with an HCT-CI score ≥3 had a significant worse OS (HR 2.5; P=0.024) and RFS (HR 2.5; P=0034). Those recipients who received grafts from 9/10 MUD and haploidentical donors had a significant worse RFS (HR 2.1; P=0.049). Disease risk index was not a significant risk factor for OS or RFS. Lastly, those recipients who received grafts from haploidentical donors had a significant worse GRFRS (HR 2.9; P=0.04).
Conclusion
The dose of ATG for GVHD prophylaxis is not well established. The combination of ATG (2mg/kg), PTCy, and CsA is safe and provides an effective acute GVHD prophylaxis in PB RIC allo-HSCT.
Further investigations are required to analyze the effect of this prophylaxis in chronic GVHD to better state long-term outcome conclusions and to improve post-transplant outcomes in those recipients who received grafts from mismatched donor sources (haploidentical and 9/10 matched unrelated donors).
Michelis:CSL Behring: Other: Financial Support. Mattsson:Celgene: Honoraria; Therakos: Honoraria; Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.