Background: Salvage autologous stem cell transplant (SAT)is an alternative treatment option for relapsed multiple myeloma patients that offers additional progression-free survival (PFS2) and overall survival (OS2) advantage over salvage chemotherapy. We conducted a meta-analysis to evaluate the outcomes of salvage transplant in patients with relapsed multiple myeloma after initial transplant.
Methods: This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A comprehensive literature search on PubMed, Embase, Cochrane and Web of Science was conducted up to December 31st, 2018. Two independent reviewers screened the literature and extracted data. All studies including randomized, retrospective or prospective studies in multiple myeloma patients who underwent salvage autologous transplant were included. Abstracts, posters, review articles, case reports and studies with syngeneic and tandem transplant were excluded. Articles were excluded if they did not provide transplant related outcomes data. The search terms included "Salvage autologous stem cell transplantation", "Second autologous stem cell transplantation", "multiple myeloma". 'Meta' and "Metafor' libraries in R software (CRAN Project) were used for the analysis. Pooled estimates and 95% confidence intervals were calculated using DerSimonian-Laird (DL) random effects model. Heterogeneity between studies was evaluated using Q test and sensitivity analysis.
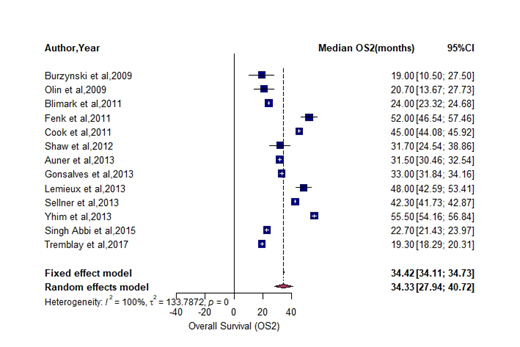
Results: The search strategy identified over 3260 articles; 16 studies (n = 1113 patients; 1 randomized trial; 15 retrospective studies) were selected for this meta-analysis. The sample size of the studies varied between 25 and 200 patients. All studies used melphalan conditioning for salvage transplant. A significant number of patients in about 10 studies received maintenance after initial transplant. Only one study included patients who received maintenance therapy after salvage transplant. Pooled rate of patients achieving partial response or more(≥PR) after salvage transplant was 76% (95%CI: 68-83; I2=84%). Pooled rate of transplant related mortality (TRM2) was 5.5% (95%CI: 2.6-9.3; I2=78%). The pooled estimates showed a median progression free survival (PFS2) 13.5 months (95%CI: 11.3 - 15.6; I2=100%), overall survival (OS2) 34.3 months (95%CI: 27.9 - 40.7; I2=100%). The results are shown in figures 1&2.
Conclusion: SAT approach had favorable outcomes of achieving durable PFS and OS in relapsed myeloma patients. A Higher TRM was observed with salvage transplant than in upfront transplant. Prospective randomized trials are needed to define benefits of SAT in comparison with "best non-ASCT" therapy in patients with MM who relapse after primary therapy.
Mansour:Abbvie: Other: Stock; Astra Zeneca: Other: Stock; Bluebird Bio: Other: Stock; CRISPR: Other: Stock; Editas: Other: Stock; Johnson and Johnson: Other: Stock; Novartis: Other: Stock.
Author notes
Asterisk with author names denotes non-ASH members.