Crypt base intestinal stem cells (ISCs) marked by Lgr5 and Olfm4 maintain the intestinal epithelium, and Paneth cells (PCs) provide an epithelial niche for ISCs in the small bowel. ISCs are reduced during gastrointestinal (GI) GVHD, but the precise mechanisms including the role of niche injury are unknown. Additionally, the specific effects of Interferon-γ (IFNγ) on intestinal epithelium in GVHD remain ill-defined.
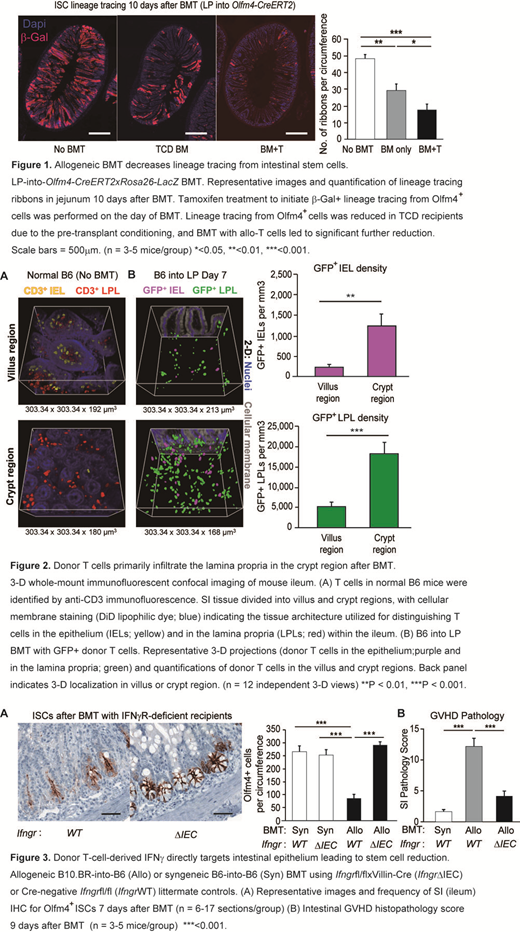
We evaluated kinetics of ISC loss by histology after allogeneic (allo) BMT using Lgr5-LacZ reporter mice. In both MHC- and miHA-mismatched models (LP>B6 and B6>BDF1), ISC numbers quickly recovered from pretransplant TBI conditioning in recipients of T-cell-depleted (TCD) BMT by day +10, but ISCs failed to recover in recipients of allo T cells. T-cell-induced ISC reduction was functionally validated by genetic marking of stem cell progeny and by culturing intestinal organoids from crypts isolated post-BMT. Similar to the kinetics of ISC loss, ISC-dependent organoid-forming capacity was impaired in recipients of allo T cells compared with TCD BMT recipients on day +10 (p<0.05). Likewise, BMT into Olfm4 reporter mice showed significantly reduced lineage tracing from ISCs in recipients of allo T cells (Fig. 1).
To better understand the potential for T cells to interact with the ISC compartment, we performed whole-mount 3-D microscopy to distinguish T cell localization within the intraepithelial and lamina propria compartments post-transplant. We found that donor T cells invading the small bowel after BMT were mostly in the crypt region, and infiltration within the lamina propria adjacent to the ISC compartment was much greater than invasion within the epithelium itself (Fig. 2).
We next established an ex vivo co-culture system, to model interactions between intestinal epithelium and donor T cells and investigate mechanisms of T-cell-mediated ISC injury. Screening of effector pathways revealed no impact of perforin or FasL, but identified IFNγ as a principal mediator of ISC injury. Culture with allo T cells significantly reduced viable human and mouse intestinal organoid numbers, and this was inhibited by IFNγ neutralization. IFNγ receptor knockout (IFNγR-/-) organoids were resistant to T cells. IFNγ increased expression of Bak1 and decreased expression of Bcl2 in organoids, and induced ISC apoptosis defined by Annexin+Dapi-Lgr5+ phenotype. ISC killing was mediated by intraepithelial JAK/STAT signaling, as JAK1- and STAT1-deficient organoids were resistant, and it was inhibited by Ruxolitinib.
Investigating the role of IFNγ in vivo, FACS analysis confirmed donor T cells to be the primary producers of IFNγ in crypt lamina propria, and BMT with IFNγ-/- donor T cells reduced crypt apoptosis, and preserved ISC frequencies. Moreover, BMT with recipients lacking IFNγR specifically in the intestinal epithelium significantly protected ISCs, reduced crypt apoptosis, and ameliorated GI GVHD pathology (Fig.3). Furthermore, ISCs were also protected by epithelial deletion of STAT1 and by Ruxolitinib treatment.
As specific genetic manipulation of ISCs in vivo is not possible because genetic targeting of ISCs results in the same changes in their progeny, we utilized ex vivo models to determine if IFNγ kills ISCs by directly inducing their apoptosis or by damaging the PC niche. Manipulation of the niche by culturing wild-type ISCs with IFNγR-/- PCs was not protective to allo T cells. Using a niche-independent high-purity Lgr5+ ISC culture system based on combined GSK3β and HDAC inhibition, IFNγ directly induced cleaved-caspase-3+ ISC apoptosis and substantial ISC colony death, which were inhibited by Bak/Bax deficiency and by the pan-caspase inhibitor QVD. These results confirmed that IFNγ can directly induce ISC apoptosis independent of other cytotoxic effector molecules and independent of injury to the PC niche.
In summary, T cells migrating to the GI tract primarily infiltrated the lamina propria adjacent to the ISC compartment, and T-cell-derived IFNγ directly targeted intestinal epithelium via JAK1/STAT1 signaling to induce ISC apoptosis in a PC-independent manner. ISC reduction and GI GVHD pathology were prevented by inhibiting the IFNγR/JAK1/STAT1 axis within the intestinal epithelium, indicating that in addition to their effects on T cells, JAK inhibitors may treat GVHD by inhibiting pathologic cytokine signaling within target organs and shielding them from allo T cells.
Hanash:Nexus Global Group LLC: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.