Background: The JAK2/ STAT pathway has been implicated in the survival and proliferation of the leukemic stem-cell in chronic myeloid leukemia (CML). BCR-ABL mediates the activation of STAT3 and STAT5, inhibiting stem-cell differentiation. Activation of JAK1 and JAK2 leads to STAT3 phosphorylation and has been associated with tyrosine kinase inhibitors (TKI) resistance. Thus, the inhibition of JAK2/STAT pathway and nonreceptor tyrosine kinase (TYK2) could increase the sensitivity to TKIs activity and potentially eradicate early progenitors. The JAK1/2 inhibitor ruxolitinib, approved for the treatment of myelofibrosis, has also moderate inhibitory activity against TYK2. This Phase I/II study aims to determine the efficacy of ruxolitinib and TKI activity in CML with residual disease.
Methods: This is a single-arm Phase I/II trial of Ruxolitinib in combination with TKI for CML with minimal residual disease while on TKI therapy. Eligible patients (pts) included adults 18 years and older, on imatinib therapy for a minimum of 18 months with no dose modifications in the last 6 months. Patients with complete hematologic response (CHR) but without a major molecular response (MMR) or complete cytogenetic response (CCyR) or with loss of MMR at any time were included. ECOG performance status 0-2 and adequate organ function was required. Patients on therapy for at least 2 years without MMR or 5 years without a CMR (i.e., undetectable transcripts, ≥100,000 copies ABL) were included. Patients on TKI after alpha-interferon (INF) or prior TKI failure were included. Ruxolitinib was given at a dose of 5 mg BID, 10 mg BID, and 15 mg BID daily in 28 days cycles for up to 2 years. The primary endpoint was to determine the dose-the limiting toxicity and maximal tolerated dose of the combination. Secondary endpoints were to determined safety and clinical activity, and the effect on molecular responses by DNA PCR.
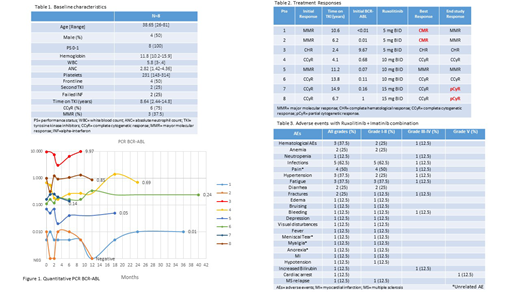
Results: A total of 8 pts with chronic phase-CML and detectable BCR-ABL transcript levels were enrolled between November 2013 and July 2018. The median age was 38.6 years [26-81]. Four pts (50%) were male. 2 pts (25%) had failed INF, and 2 pts (25%) had received one prior TKI (1 pt failed dasatinib, and 1 pt discontinue nilotinib due to adverse effects). The imatinib dose was 300 mg (n=1) and 400 mg (n=7). The median time on TKI therapy was 8.6 years [2.4-14.8]. At baseline 7 pts (87.5%) had a CCyR, and 3 pts (37.5%) had achieved a MMR. Baseline characteristics are listed in Table 1.
The median time on study was 11.4 months. 2 pts achieved CMR after 1 and 12 months on therapy respectively, 5 pts had stable disease (1 pt in MMR and 4 pts in CCyR), and 1 pt had no response, with persistent BCR-ABL PCR above 2%. (Table 2) The median time to best response was 1 month [1-25]. Molecular responses by DNA PCR are illustrated in Figure 1.
The treatment was well tolerated, with mostly grade I-II adverse events (AEs). There were no dose-limiting toxicities. The most common non-hematological adverse events (AEs) were infections (62.5%), pain (50%), fatigue (37.5%), hypertension (25%) and diarrhea (25%) of grade I-II. The most frequent hematological AE's were grade I-II anemia (25%) and grade III-IV neutropenia (12.5%). Adverse events are listed in Table 3.
With a median follow-up of 44.7 months, 1 pt did not respond and died during treatment from an unknown cause, and 2 pts lost CMR after 2 and 7 months respectively. Of the 5 pts with SD, 2 pts lost the CCyR after 5 and 6 months. Overall, 1 patient remains on the study and 7 pts discontinued treatment, including 2 pts that withdrew consent due to adverse effects (grade II fatigue),1 pt due to death, 1 pt due to lost of response and 3 pts for persistence of BCR-ABL transcripts.
Conclusions: The ruxolitinib and imatinib combination was safe and well-tolerated in CP-CML, with an acceptable AE profile; with most toxicities limited to grade I-II AE. Nonetheless, the incidence of a CMR was 25% (2 pts) with a short duration of response. There was no apparent clinical benefit seen with the combination. The study is no longer recruiting patients. (NCT01751425)
Kantarjian:Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Research Funding; Takeda: Honoraria; Ariad: Research Funding; BMS: Research Funding; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding; Jazz Pharma: Research Funding; Pfizer: Honoraria, Research Funding; Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Cyclacel: Research Funding; Astex: Research Funding; Novartis: Research Funding. Borthakur:GSK: Research Funding; BioTheryX: Membership on an entity's Board of Directors or advisory committees; AbbVie: Research Funding; Cyclacel: Research Funding; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; NKarta: Consultancy; PTC Therapeutics: Consultancy; Oncoceutics, Inc.: Research Funding; Eli Lilly and Co.: Research Funding; BMS: Research Funding; AstraZeneca: Research Funding; Bayer Healthcare AG: Research Funding; Agensys: Research Funding; Oncoceutics: Research Funding; Novartis: Research Funding; Xbiotech USA: Research Funding; Eisai: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Strategia Therapeutics: Research Funding; Polaris: Research Funding; Arvinas: Research Funding; Merck: Research Funding; Cantargia AB: Research Funding; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Argenx: Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Incyte: Research Funding. Verstovsek:Gilead: Research Funding; Roche: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Promedior: Research Funding; CTI BioPharma Corp: Research Funding; Genetech: Research Funding; Blueprint Medicines Corp: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Research Funding; Pharma Essentia: Research Funding; Astrazeneca: Research Funding; Ital Pharma: Research Funding; Protaganist Therapeutics: Research Funding; Constellation: Consultancy; Pragmatist: Consultancy; Incyte: Research Funding. Ravandi:Macrogenix: Consultancy, Research Funding; Selvita: Research Funding; Menarini Ricerche: Research Funding; Cyclacel LTD: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Consultancy, Research Funding. Jabbour:Pfizer: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Cyclacel LTD: Research Funding. Cortes:Biopath Holdings: Consultancy, Honoraria; Takeda: Consultancy, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Sun Pharma: Research Funding; Merus: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy.
Ruxolitinib, a JAK1/2 inhibitor, is approved for myelofibrosis and polycythemia vera refractory or intolerant to hydroxyurea. In this trial ruxolitinib is used in combination with TKI for minimal residual disease in CML
Author notes
Asterisk with author names denotes non-ASH members.