Introduction: Tyrosine kinase inhibitors (TKIs) represent the standard of care for patients with chronic-phase chronic myeloid leukemia (CP-CML). The emergence and availability of second-generation TKIs (dasatinib, nilotinib and bosutinib) has led to increased choice for patients and physicians, with data from randomized clinical trials indicating improved efficacy of such therapies compared to the first-generation TKI (imatinib). Differences in the efficacy of second-generation TKIs are negligible; however, all have distinct dosing instructions and safety profiles.
Understanding the patient perspective is invaluable for optimizing healthcare decisions, with interest in information regarding patient preferences growing among a wide variety of stakeholders, including regulatory agencies, reimbursement bodies and prescribers. However, evidence is lacking regarding treatment preferences and priorities for patients with CP-CML.
The aim of this study was to explore attributes of currently-licensed TKI therapies that are relevant and important to patients with CP-CML. This research represents the first step in the conduct of a discrete choice experiment (DCE), or conjoint analysis, to explore treatment preferences more broadly among CP-CML patients. Patient-guided design is crucial to ensuring attributes of investigation are meaningful. Hence the qualitative insights will advise and validate attribute and level selection for inclusion in the forthcoming DCE.
Methods: In-depth qualitative interviews were conducted with 12 US-based patients with a clinician-confirmed diagnosis of CP-CML. The sample included 10 females and 2 males (median age 46 years) who were diagnosed 2 years (n=3), 4-7 years (n=5), 10-12 years (n=3) and 16.5 years (n=1) prior to interview. Ten patients had received a second-generation TKI. Current treatments were dasatinib (n=8), bosutinib (n=1) and imatinib (n=3). Previous treatments included imatinib (n=6), nilotinib (n=3) and dasatinib (n=1).
The interviews utilized concept elicitation techniques to capture attributes of importance in both TKI treatment experience and TKI treatment selection. Transcripts were thematically analyzed using Atlas.ti.
Results: Open-ended questioning elicited an array of attributes related to TKI treatments.
It was important to all patients to ensure that a CP-CML treatment was effective. Treatment efficacy was conveyed with multiple descriptors; most commonly "BCR-ABL" (n=7) or major molecular response "MMR", polymerase chain reaction "PCR" and "remission" (each n=3).
Eight patients discussed the importance of dose frequency, specifically the burden of scheduling self-administration of oral tablets (n=5). Seven patients conveyed the difficulty of taking a TKI with specific meal requirements, most notably when fasting is required prior to and post-administration (n=4).
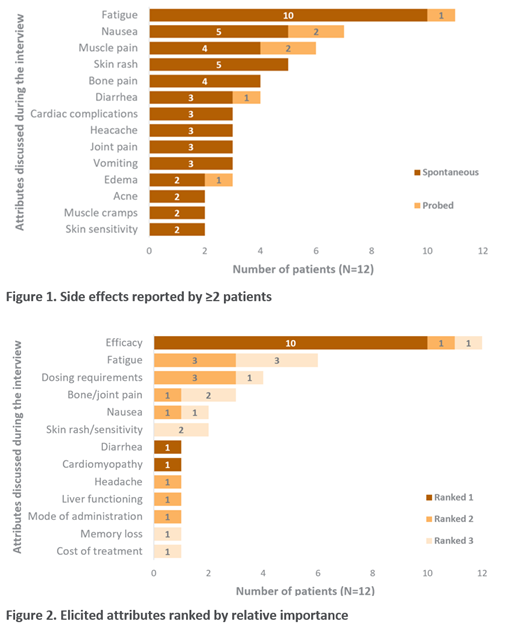
A multitude of important treatment side effects were elicited at investigation into the patient TKI treatment experience. Fatigue was reported by 11 patients (n=10 spontaneous) and considered by most patients (n=8/11) to be directly attributable to treatment versus the CML itself. Four patients considered fatigue an inevitable component of TKI treatment. Nausea was elicited by 7 patients (n=5 spontaneous) and some patients (n=5/9) considered it to be influential in treatment selection. Muscle pain was reported by 6 patients (n=4 spontaneous), while skin rash was reported by 5 patients and bone pain by 4 patients (all spontaneous). Diarrhea was reported by 4 patients (n=3 spontaneous). Additional symptoms reported by ≤2 patients are presented in Figure 1.
Spontaneously elicited concepts were ranked by importance. All patients considered efficacy within the top three most important attributes. Six patients considered fatigue to be either of second or third most-importance. The rankings in Figure 2 serve to portray the varied perspectives and experiences of patients on TKI treatments.
Conclusions: The TKI treatment experience for CP-CML patients is multifaceted. This type of study - the first that we know of - supplies valuable insight into the patient perspectives of TKI treatment to establish a foundation to better inform relevant decisions in the field. The qualitative findings will provide the input necessary to inform attribute and level selection to characterize treatment profiles ahead of an upcoming patient-preference DCE.
Mason:Adelphi Values: Employment; Pfizer: Consultancy. Mamolo:Pfizer: Employment, Equity Ownership. Johnson:Pfizer Inc: Consultancy; Adelphi Values Ltd: Employment. Viqueira:Pfizer Inc: Employment, Equity Ownership. Gater:Pfizer Inc: Consultancy; Adelphi Values Ltd: Employment. Russell-Smith:Pfizer: Employment, Equity Ownership. Tatlock:Adelphi Values: Employment. Shah:Pfizer: Employment, Equity Ownership. Cappelleri:Pfizer: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.