Background: Chimeric transcripts are frequent genetic abnormalities in hematological malignancies that often contribute to leukemogenesis and play crucial roles for risk stratification, MRD monitoring and targeted therapy. However, current standard techniques for detection of gene fusions (chromosome banding analysis (CBA) and fluorescence in situ hydridization (FISH)) are only able to predict known and/or non-cryptic gene fusions. RNA sequencing (RNAseq) has successfully been applied for determination of genomic gene fusions.
Aim: Comprehensive analysis of fusion genes in AML (acute myeloid leukemia) and MDS (myelodysplastic syndrome) patients and evaluation of the beneficial use of RNAseq in detecting recurrent and novel fusions.
Methods: RNAseq was performed for 579 AML and 630 MDS patients for the detection of recurrent and novel fusion transcripts. WGS (whole genome sequencing) and cytogenetics (CBA, FISH) were applied for validation of the respective transcripts. 151 bp paired-end reads were produced on a NovaSeq 6000 system (Illumina, San Diego, CA) with a yield between 35 and 125 million paired reads per sample. Reciprocal fusion transcripts were counted as one fusion event. All reported p-values are two-sided and were considered significant at p<0.05.
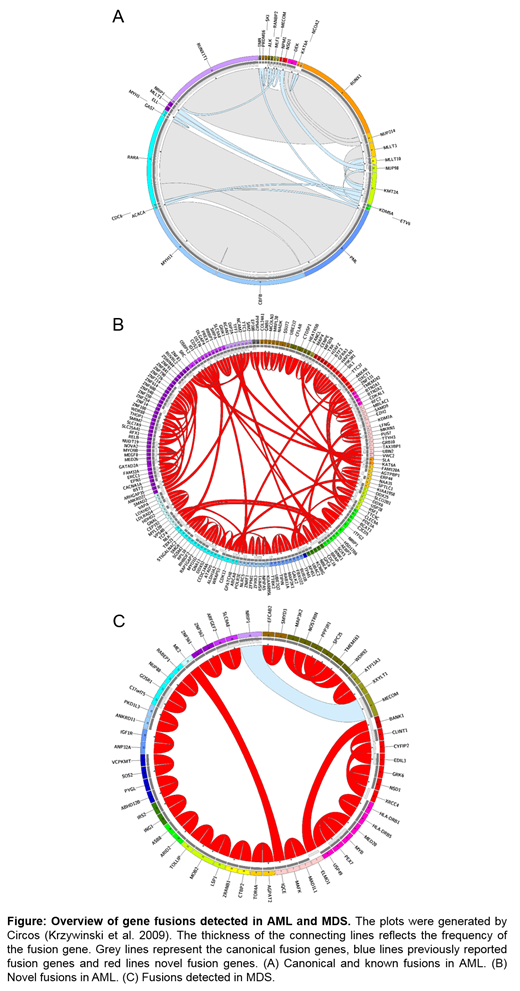
Results: After stringent filtering and validation by WGS and/or cytogenetics, for AML, 279 fusion events (corresponding to 147 unique gene fusions) were detected in 208 cases (36% of patients). 213/279 (76%) were confirmed by WGS and cytogenetics, 54/279 cases (19%) by WGS only and 12/279 (4%) cases by cytogenetics only. As a proof of principle, entity-defining rearrangements were detected most frequently: RUNX1-RUNX1T1 (n=42), CBFB-MYH11 (n=39) and PML-RARA (n=39). Other recurrent fusions included KMT2A-MLLT3 (n=6), KMT2A-MLLT10 (n=3) and DEK-NUP214 (n=4) (Fig 1A). However, in addition a high number (130/279, 47%) of so far unreported gene fusions were detected, including 49/130 inter-chromosomal and 81/130 intra-chromosomal fusions. These comprised 92/130 cases for which both fusion partners have not been reported before and 38/130 cases with a novel partner of a gene previously reported in hematological malignancies, including novel partners for NUP98 (XRN1), ETV6 (FAAP100, ARNTL2, SMCO2), RUNX1 (THOC6, EIF3E, OPHN1, TMEM50B), RARA (CCDC33), CBFB (TTC3, HMGB1) and KMT2A (NCBP1, ARHGEF12) (Fig 1B) (all validated by WGS and/or cytogenetics (see above)). Most of the novel fusions were detected only once, however two of them were observed in two patients each (NRIP2-ITFG2, CTDSP1-CFLAR). Moreover, cases with novel fusions were characterized by a very high frequency of TP53 mutations (59% vs. 1% in cases with known fusions and 9% in cases with no detected fusions, p<0.001), whereas FLT3-ITD and NPM1 mutations were rather rare (NPM1: 8% (novel) vs. 0% (known) vs. 35% (no fusion); FLT3-ITD: 3% (novel) vs. 14% (known) vs. 24% (no fusion). Consequently, a large number of cases with novel fusions depicted a complex karyotype (62% (novel) vs. 1% (known) vs. 9% (no fusion)). Regarding age, patients with known fusions were significantly younger than patients with novel fusions or without detected fusions (median age: 54 years (known) vs. 72 (novel) vs. 70 (no fusion), p<0.001).
For MDS, 30 fusions (29 unique transcripts) were observed in 28/630 cases (4% of all patients). 4/30 (13%) of detected gene fusions were inter-chromosomal, while the majority (26/30, 87%) was intra-chromosomal. 27/30 (90%) fusions were validated by WGS, 9/30 (30%) by CBA, comprising 6/30 (20%) cases that were validated by both methods. Only one of the detected fusions was detected in >1 patient (n=2) and was described previously (MECOM-NRIP1), all others (n=28) are so far unreported. Of these, 23/28 comprise both so far undescribed fusion partners, whereas for 5/28 fusions one partner was previously reported to function in hematological neoplasms (e.g. MYB-PEX7, RABEP1-NUP88).
Conclusions: (1) A large number of novel fusions were detected by RNAseq and validated by WGS and cytogenetics, especially in AML. (2) These novel fusions correlate with a very high frequency of TP53 mutations, their pathogenic role has to be evaluated further. (3) This data may provide the basis for identifying potential new actionable targets (e.g. for personalized vaccine or adoptive cell-based therapy development) and developing markers for patient sensitive MRD monitoring.
Stengel:MLL Munich Leukemia Laboratory: Employment. Walter:MLL Munich Leukemia Laboratory: Employment. Meggendorfer:MLL Munich Leukemia Laboratory: Employment. Kern:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.