Background
Primary immune thrombocytopenia (ITP) is an acquired autoimmune bleeding disorder, characterized by a low platelet count (<100×109/L) in the absence of other causes. In most patients, IgG autoantibodies are detected which accelerate platelet clearance, inhibit platelet production, induce platelet apoptosis or complement-dependent lysis, and may alter platelet function, resulting in increased risk of bleeding and impaired quality of life. Efgartigimod is a human IgG1 antibody Fc-fragment, a natural ligand of the neonatal Fc receptor (FcRn), engineered for increased affinity to FcRn whilst preserving characteristic pH-dependent binding. Efgartigimod blocks FcRn, preventing IgG recycling, causing targeted IgG degradation. In healthy volunteers (NCT03457649), efgartigimod was well tolerated and induced a rapid reduction of total IgG and all IgG subtypes. Targeted reduction of autoantibodies through FcRn blockade may prevent their pathogenic actions and represents a novel treatment modality in ITP. We investigated the safety and efficacy of efgartigimod in adult patients with primary ITP in a randomized, double-blinded, placebo-controlled Phase 2 study (NCT03102593).
Methods
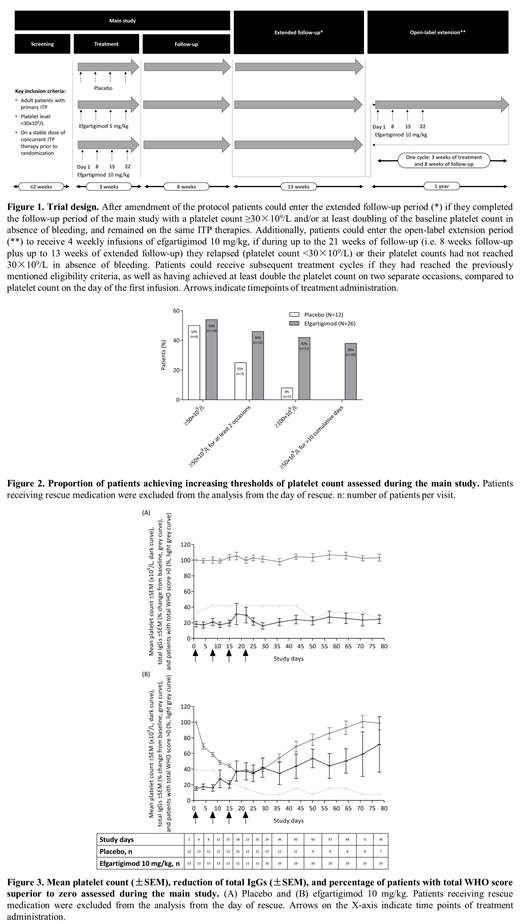
After a 2-week screening period, patients were randomized 1:1:1 to receive 4 weekly intravenous infusions of either placebo or efgartigimod at a dose of 5 mg/kg or 10 mg/kg. Following periods included 8-week follow-up, up to 13 weeks extended follow-up, and a 1-year open-label extension (OLE) where patients could be treated with efgartigimod 10 mg/kg (Figure 1). Patients aged 18 to 85 years with confirmed primary ITP, and an average of 2 platelet counts ˂30×109/L during screening (with no single reading >35×109/L), were recruited. Oral corticosteroids, oral immunosuppressants, and/or thrombopoietin receptor agonists at stable doses were permitted.
The primary endpoint was safety. Secondary endpoints included pharmacodynamic (PD) markers, pharmacokinetic (PK) parameters, presence of anti-drug antibodies (ADA) and autoantibodies, and efficacy. Data for all endpoints were summarized by group using descriptive statistics. Data until the last visit of the first treatment cycle of the OLE period are reported.
Results
Thirty-eight patients (placebo [N=12], efgartigimod at a dose of 5 mg/kg [N=13] or 10 mg/kg [N=13]), mostly with longstanding ITP (median disease duration 4.8 [0.1-47.8] years) who had insufficient response to prior ITP therapy or failed splenectomy (N=6), were randomized. Twenty (52.6%) patients had a baseline platelet count <15×109/L.
Efgartigimod was well tolerated with no dose-related safety observations and the safety profile was consistent with previous observations in healthy volunteers. No increased risk of infection was apparent. Low positive pre- and post-dose ADA titers were measured in all groups and did not have an apparent effect on PK/PD parameters.
Treatment with efgartigimod resulted in a rapid reduction of total IgG (up to 63.7% mean change from baseline) and all IgG subtypes in all treated patients. Autoantibodies were identified in all patients and were reduced following efgartigimod treatment.
Efgartigimod-treated groups achieved a higher maximum mean platelet count change from baseline compared to the placebo group. A higher proportion of efgartigimod-treated patients achieved a platelet count of ≥50×109/L and ≥100×109/L at any time compared to the placebo group (Figure 2). Forty-six percent patients on efgartigimod vs. 25% on placebo achieved a platelet count of ≥50×109/L on at least 2 occasions and 38% vs. 0% achieved ≥50×109/L for at least 10 cumulative days. A decreased incidence of bleeding, measured using WHO and ITP-BAT scales, was observed in both efgartigimod-treated groups.
In the OLE period, in which 12 patients received efgartigimod 10 mg/kg, 8 (66.7%) patients achieved platelet count ≥50×109/L on at least 2 occasions.
Conclusion
A short 3-week treatment cycle of efgartigimod in patients with ITP predominantly refractory to previous lines of ITP therapy was well tolerated, markedly reduced IgG levels, was associated with clinically relevant increases in platelet counts, and reduced the proportion of patients with bleeding (Figure 3). This suggests that targeted IgG reduction with efgartigimod is a potential new treatment modality in primary ITP, and warrants evaluation of longer-term treatment in a larger Phase 3 study.
Newland:Novartis: Consultancy, Honoraria, Research Funding; Angle: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Ono: Consultancy, Honoraria; Shionogi: Consultancy, Honoraria; Rigel: Consultancy, Honoraria, Research Funding; Argenx: Consultancy, Honoraria; Dova: Consultancy, Honoraria. Sánchez-González:Amgen: Consultancy, Speakers Bureau; Gilead: Speakers Bureau; Navartis: Consultancy, Speakers Bureau; Shire: Speakers Bureau; Takeda: Consultancy, Speakers Bureau. Godar:Argenx: Employment. Verschueren:Argenx: Consultancy. Gandini:Argenx: Employment, Equity Ownership. Ulrichts:Argenx: Employment. Beauchamp:Argenx: Employment. Dreier:Argenx: Employment. Ward:Argenx: Equity Ownership, Honoraria, Research Funding. Michel:Rigel: Consultancy; Amgen: Consultancy; Novartis: Consultancy. Liebman:Pfizer: Consultancy; Dova: Consultancy; Bristol-Myers: Research Funding; Argenx: Consultancy; Rigel: Consultancy; Janssen: Research Funding; Novartis: Consultancy. De Haard:Argenx: Employment, Equity Ownership, Patents & Royalties. Leupin:Argenx: Employment, Equity Ownership, Patents & Royalties. Kuter:Dova: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; UCB: Consultancy, Honoraria; Momenta: Consultancy, Honoraria; Caremark: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Protalix: Consultancy, Honoraria; Principia: Consultancy, Honoraria, Research Funding; Platelet Disorder Support Association: Consultancy, Honoraria; Shire: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Principia: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria; Kyowa-Kirin: Consultancy, Honoraria; Kyowa-Kirin: Consultancy, Honoraria; Merck Sharp Dohme: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Merck Sharp Dohme: Consultancy, Honoraria; Protalix: Consultancy, Honoraria; Rigel: Consultancy, Honoraria, Research Funding; Rigel: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Bristol Myers Squibb (BMS): Consultancy, Honoraria, Research Funding; Alnylam: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria; Protalex: Consultancy, Honoraria, Research Funding; Dova: Consultancy, Honoraria; Protalex: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Up-to-Date: Consultancy, Honoraria, Patents & Royalties: 3 Up-to-Date chapters; Up-to-Date: Consultancy, Honoraria, Patents & Royalties: 3 Up-to-Date chapters; Zafgen: Consultancy, Honoraria; Takeda (Bioverativ): Consultancy, Honoraria, Research Funding; Platelet Disorder Support Association: Consultancy, Honoraria; Shinogi: Consultancy, Honoraria; Agios: Consultancy, Honoraria, Research Funding; Actelion (Syntimmune): Consultancy, Honoraria, Research Funding; Takeda (Bioverativ): Consultancy, Honoraria, Research Funding; Caremark: Consultancy, Honoraria; Kezar: Research Funding; Kezar: Research Funding; Shire: Consultancy, Honoraria; Argenx: Consultancy, Honoraria, Research Funding; Alnylam: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb (BMS): Consultancy, Honoraria, Research Funding; Argenx: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Actelion (Syntimmune): Consultancy, Honoraria, Research Funding; Shinogi: Consultancy, Honoraria; Zafgen: Consultancy, Honoraria; Momenta: Consultancy, Honoraria; UCB: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.