In China, it is estimated that there are over 50,000 patients with β-thalassemia major, of which the standard care requires regular red blood cell transfusions along with iron chelation, often associated with iron overload and organ damage. The only curative therapy, allogeneic hematopoietic stem cells (HSCs) transplantation, is primarily available to a fraction of young patients with HLA-matched sibling donors. A study has estimated that 1% of patients with β-thalassemia major in China expect to live beyond age 20, representing severe unmet needs. Recently, transplantation of genetically modified autologous HSCs is emerging as an attractive therapeutic alternative for β-thalassemia. Epidemiology data have shown that in some patients with β-thalassemia, manifested clinical symptoms could be ameliorated by elevated fetal hemoglobin (HbF). As BCL11A is one of the main transcription factors known to play critical roles to repress expression of γ-globin, a critical element of HbF, we are developing ET-01, in which autologous CD34+ cells are edited by CRISPR/Cas9 to disrupt the BCL11A erythroid enhancer and reactivate the developmentally silenced γ-globin, to treat patients with β-thalassemia. Our data have shown that in ET-01, the BCL11A enhancer can be edited with high precision and efficiency, resulting in HbF levels above a potential therapeutic threshold. We have developed and scaled up the manufacturing process under the cGMP standard. IND-enabling preclinical studies, including GLP studies using ET-01 manufactured at the clinical scale, are on-going. Overall our data support continuing development of ET-01 as a potentially safe and efficacious therapy for patients with β-thalassemia major
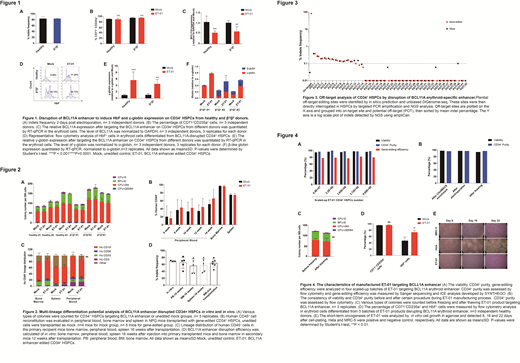
To disrupt the BCL11A enhancer, Cas9 mRNA and synthetic sgRNA were delivered into CD34+ HSCs from healthy and β0/β0 donors using electroporation. Indels frequencies (i.e., gene editing efficiency) were approximately 80%, consistent for both types of donors. Efficiency of in vitro erythroid differentiation was essentially identical between ET-01 and unedited CD34+ HSCs. HbF+ cells and γ-globin mRNA levels were both significantly elevated in ET-01 compared to unedited CD34+ HSCs. Importantly, in ET-01 made from cells of β0/β0 donors, the level of γ-globin, plus that of β-globin, reached above a threshold with potentially clinically meaningful effect. Meanwhile, data from the colony-forming unit assay have shown colony types and total numbers of colonies were comparable between those of ET-01 and unedited CD34+ HSCs, suggesting that the colony-forming capability was not significantly affected in ET-01.
To evaluate the specificity of gene-editing methodology used in ET-01, we generated a signature panel consisting of 42 sites with highest probability of off-target editing by in silico prediction and unbiased DiGenome-seq. These sites were deeply interrogated via targeted PCR amplification and next generation sequencing analysis. The data showed no significant off-target events at these sites, suggesting minimal off-target effects for ET-01.
Moreover, to evaluate the in vivo engraftment potential of ET-01, we transplanted ET-01 and unedited CD34+ HSCs into immunodeficient NPG mice. Both types of cells produced similarly rapid and efficient engraftment in all mice, and similar multi-lineage reconstitution of human cells in multiple hematopoietic and immune organs. Importantly, the Indels efficiencies of ET-01 were maintained at similar levels in NPG mice after 4 months transplantation and after 2nd transplantation. These findings suggest that ET-01 retained in vivo long-term hematopoietic repopulating and differentiation capabilities. No tumorigenicity was observed in these in vivo studies.
ET-01 manufacturing process has been successfully scaled up to 9.69x108 CD34+ HSC produced using mobilized leukopak from healthy donors. This upper bound is limited by the amount of starting materials obtained, not the actual process capacity. High cell viability, CD34+ purity and Indels frequencies were maintained throughout the manufacturing process and achieved in all batches. In vitro erythroid differentiation efficiency, HbF+ cells and γ-globin mRNA levels were similar to those observed at research scale. IND-enabling preclinical studies, including GLP studies using ET-01 manufactured at the clinical scale, are ongoing.
Fang:EdiGene Inc.: Employment. Yuan:EdiGene, Inc.: Employment. Yu:EdiGene, Inc.: Employment. Yang:EdiGene, Inc.: Employment. Liu:EdiGene Guangzhou Inc.: Employment. Shi:EdiGene Guangzhou Inc.: Employment. Zhang:EdiGene Inc.: Employment. Zhang:EdiGene Inc.: Employment. Zhao:EdiGene Inc.: Employment. Li:EdiGene Inc.: Employment. Wei:EdiGene, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.