Key Points
SUVmax did not predict HT in patients in the GALLIUM study.
Rebiopsy to exclude HT based on SUVmax alone may provide little benefit in de novo patients with high tumor burden FL.
Abstract
A minority of patients with follicular lymphoma (FL) undergo histological transformation (HT). This retrospective analysis of 549 patients from the phase 3 GALLIUM study (NCT01332968) assessed the relationship between maximum standardized uptake value (SUVmax) at baseline on positron emission tomography (PET) and HT risk. Previously untreated patients with high tumor burden grade 1-3a FL received obinutuzumab- or rituximab-based chemotherapy induction. The relationship between baseline SUVmax (bSUVmax) and HT risk was assessed using cutoff values for SUVmax >10 and >20. Overall, 15 of 549 (2.7%) patients with baseline PET scans experienced biopsy-confirmed HT (median follow-up, 59 months). More than 65% of patients had bSUVmax > 10, with 3.3% of these experiencing HT. Only 1 of 74 (1.4%) patients with bSUVmax > 20 underwent HT. Median bSUVmax in patients with HT vs without HT was 12.4 (range, 8.1-28.0) vs 11.8 (range, 3.1-64.4), respectively; median bSUVrange (the difference between bSUVmax of the most and least 18F-fluorodeoxyglucose–avid lymphoma sites) was 8.0 (range, 1.1-23.9) vs 7.1 (range, 0.0-59.8), respectively. There was no temporal relationship between bSUVmax and HT. Neither bSUVmax nor bSUVrange predicted HT in GALLIUM, suggesting that there may be little benefit in rebiopsy of lesions to exclude HT based on SUVmax alone before initiating therapy in patients with high tumor burden FL.
Introduction
Follicular lymphoma (FL) is considered to have an indolent clinical course, and it accounts for ∼70% of all indolent non-Hodgkin lymphomas.1 However, despite current immunochemotherapy induction and antibody maintenance, a small risk (<4% at 10 years after rituximab [R]-based treatment) remains for histological transformation (HT) into aggressive lymphoma.2
Retrospective studies have noted that nodal sites of HT may have a higher maximum standardized uptake value (SUVmax) than nontransformed sites on 18F-fluorodeoxyglucose-(FDG)–positron emission tomography (PET)/computed tomography scanning and that patients with HT typically show greater variation in SUVmax between sites.3-5 Previous small studies in mixed non-Hodgkin lymphoma subtypes suggested that SUVmax > 10 predicted aggressive lymphoma.6-8 Identifying patients at high risk for HT would allow physicians to consider treatment intensification with anthracycline-based chemotherapy.
Primary analysis of the phase 3 GALLIUM study (NCT01332968) demonstrated that investigator-assessed progression-free survival significantly improved with obinutuzumab (GA101; G)-based immunochemotherapy vs R-based immunochemotherapy (hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.51-0.85; P = .0012) in patients with previously untreated advanced FL.9 The current analysis assessed the relationship between baseline SUVmax (bSUVmax) and risk for HT in patients with FL from the GALLIUM study.
Study design
GALLIUM was a phase 3 international open-label randomized study. Eligible patients had histologically documented previously untreated CD20+ grade 1-3a FL requiring treatment, as per Groupe d’Etude des Lymphomes Folliculaires criteria. Overall, 1202 patients were randomized 1:1 to receive induction therapy with either obinutuzumab- or R-based chemotherapy (cyclophosphamide, vincristine, doxorubicin, and prednisone [CHOP]; cyclophosphamide, vincristine, and prednisone [CVP]; or bendamustine). Responders received maintenance therapy with the same antibody. Study design, patient selection, and treatment are described elsewhere.9 GALLIUM was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board or independent ethics committee of each institution. All patients provided written and informed consent.
An exploratory end point, FDG avidity using SUVmax, was assessed by an independent review committee in all patients with baseline PET scans. The data cutoff for the current analysis was February of 2018.
Assessment of PET bSUVmax values
PET scanning at baseline (implemented at screening ≤35 days prior to randomization) and end of induction was mandatory in the first 170 patients and optional thereafter. SUVmax was defined as the single voxel with the highest activity in tumor, as selected by the reviewer using MIM software (MIM Software, Cleveland, OH). Scans were assessed by investigators and an independent review committee, applying International Harmonization Project 2007 response criteria.10 The association between bSUVmax and HT was assessed using bSUVmax cutoff values > 10 and >20. Univariate analysis of baseline variables was used to calculate the HR and corresponding 95% CI and P values for the covariate effect. Covariates possibly associated with HT (using the Wald test) were entered into a backward-elimination multivariate analysis and assessed for significance (P < .05).
Results and discussion
Patient characteristics
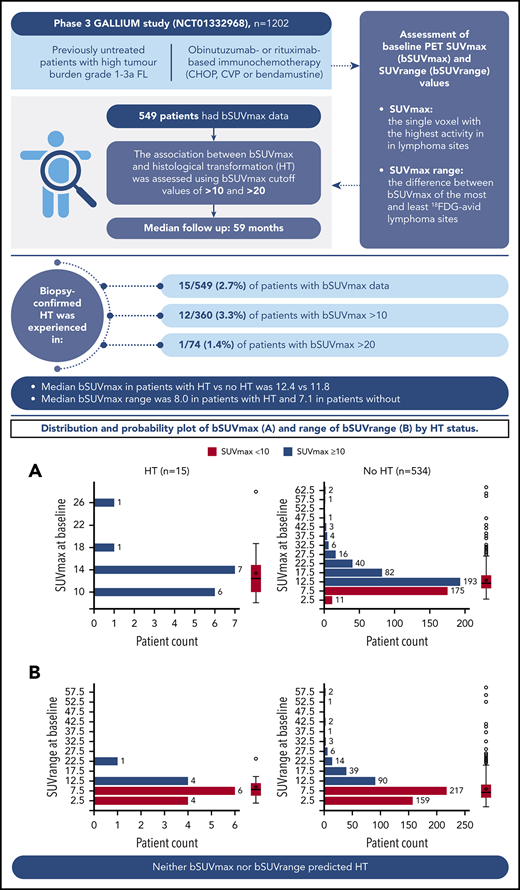
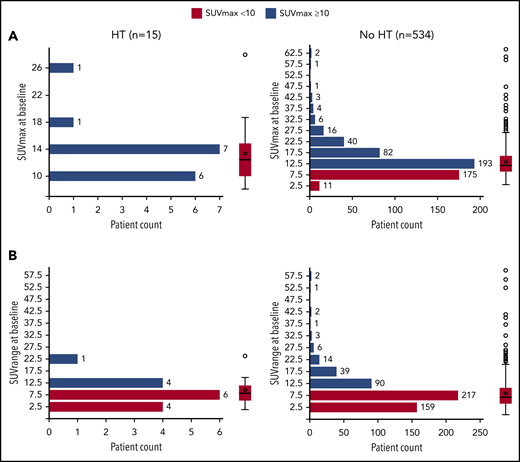
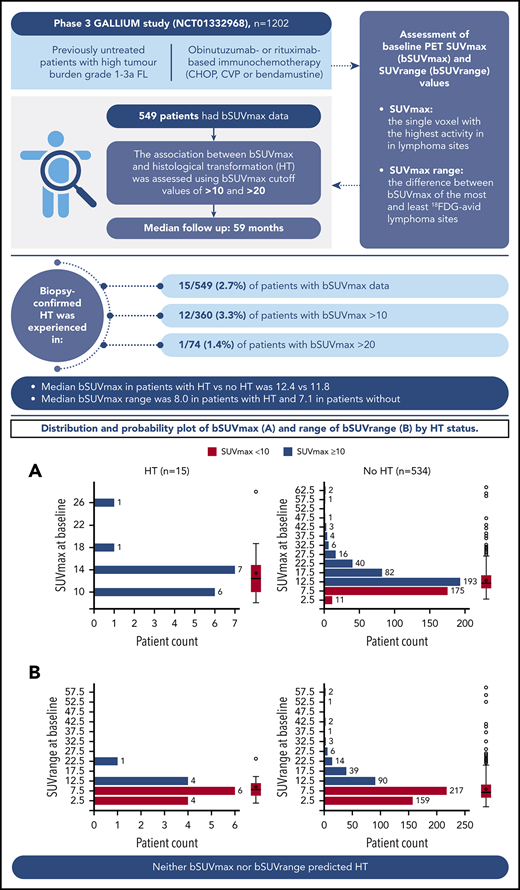
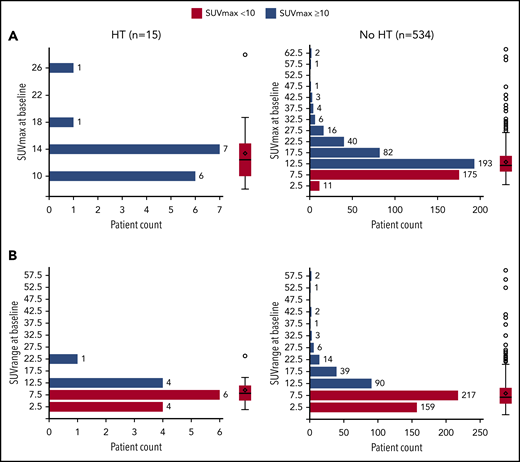
Of 549 patients with bSUVmax data (median follow-up, 59 months), 15 (2.7%) experienced biopsy-confirmed HT to diffuse large B-cell lymphoma (DLBCL) or grade 3b FL (10 DLBCL, 3 grade 3b FL, 1 DLBCL/FL, and 1 other [CD20− high-grade lymphoma with C-MYC gene fracture]). Median time to transformation was 11.1 months, and the earliest transformation was reported at 207 days (6.8 months) from randomization (supplemental Figure 1, available on the Blood Website). Of the patients who experienced HT, 9 (60.0%) received treatment with R, and 6 (40.0%) received treatment with G. Eight (53.3%) patients received bendamustine, 6 (40.0%) patients received CHOP, and 1 (6.7%) patient received CVP. Of the patients who did not experience HT, 265 (49.6%) received treatment with R, and 269 (50.4%) received treatment with G; 308 (57.7%) received bendamustine, 179 (33.5%) received CHOP, and 47 (8.8%) received CVP.
Differences in baseline characteristics were observed between patients with HT vs nontransformed patients (Table 1). Patients with HT were older and were more likely to have a poor performance status (PS) (Eastern Cooperative Oncology Group [ECOG] PS of 2), present with high-risk FLIPI (follicular lymphoma international prognostic index) and FLIPI2 scores, have a high lactate dehydrogenase (LDH) level, and have extranodal and bone marrow involvement.
Association of baseline characteristics and risk of HT
Univariate analysis of baseline characteristics showed an ECOG PS of 2, high LDH, and low hemoglobin were possibly associated with HT in patients with FL (supplemental Table 1). In a multivariate analysis of these variables, only an ECOG PS of 2 (HR, 4.55; 95% CI, 1.01-20.58; P = .049) and high LDH (HR, 4.79; 95% CI, 1.62-14.17; P = .005) significantly predicted HT. No specific chemotherapy (CHOP, CVP, or bendamustine) or antibody was significantly associated with an increased risk for HT; SUVmax was not prognostic for HT in patients treated with bendamustine, CHOP, or CVP. No association was found between depth of response at end of induction and risk of HT (P = .26).
Association between bSUVmax and HT
Overall, 360 of 549 (65.6%) patients presented with a bSUVmax > 10, with only a minority of these (12/360 [3.3%]) undergoing HT. Median bSUVmax in patients with HT vs no HT was 12.42 (range, 8.14-27.95) vs 11.77 (3.05-64.43), respectively (Figure 1), and median bSUVrange (the difference between bSUVmax of the most and least FDG-avid lymphoma sites) was 8.02 (range, 1.08-23.91) vs 7.12 (0.00-59.81), respectively. Seventy-four out of 549 (13.5%) patients had bSUVmax > 20, with only 1 of 74 (1.4%) undergoing HT. Overall, 7 of 15 (46.7%) patients with HT died.
Distribution and probability plot of bSUVmax and bSUVrange by HT status. (A) bSUVmax. (B) bSUVrange.
Distribution and probability plot of bSUVmax and bSUVrange by HT status. (A) bSUVmax. (B) bSUVrange.
Temporal relationship between bSUVmax and HT
Among patients who underwent HT (n = 15), there was no clear temporal relationship between bSUVmax and HT (supplemental Figure 1). No difference in bSUVmax was found for patients undergoing HT within 1 year vs those undergoing HT at any time point beyond 1 year (median bSUVmax, 14.36 [range, 8.14-27.95] vs 12.40 [range, 8.30-14.85], respectively; supplemental Figure 2).
Study limitations
Because patients with biopsy-confirmed HT at baseline were excluded from enrollment in the GALLIUM study, data regarding the number of those with a high SUVmax and biopsy-confirmed HT are not available. However, we report that the majority of the 74 patients who were included with bSUVmax > 20 did not transform during the study, and the earliest transformation occurred at 207 days (6.8 months). Although it is possible that the population screened included patients with de novo transformation, it is unlikely that this number would be high enough to affect the results. Additionally, only patients who underwent PET scans were analyzed; GALLIUM patients with subsequent HT but no baseline PET data were not included, thus limiting the sample size. However, the transformation rates were comparable in patients with baseline PET performed (15/549 [2.7%]) vs those without baseline PET (25/653 [3.8%]).
Conclusions
In this large PET subpopulation of the GALLIUM study, the rate of HT of FL was low (2.7%) in the 5 years after initiation of immunochemotherapy and antibody maintenance, consistent with the low rates previously reported.2
Previous smaller series including various low-grade non-Hodgkin lymphoma subtypes suggested an association between bSUVmax > 10 and HT6,7 ; however, in this much larger prospective cohort of 549 patients with previously untreated FL, bSUVmax > 10 was reported in approximately two thirds of patients and was rarely associated with early HT. The sizeable minority of patients with bSUVmax > 20 also had a very low rate of transformation. These data from a population with most patients not receiving an anthracycline are consistent with a previous study in patients with FL receiving treatment with R-CHOP, which showed that high SUVmax on FDG-PET was not predictive of clinical outcome.11
Although it is possible that SUVmax may be able to identify transformations that have already occurred, and some patients with a high SUVmax may have been excluded from the GALLIUM study because of a rebiopsy, there are few prospective data to support this practice. In a previous study, an inverse association was observed, with low SUVmax being predictive of progression in patients with high tumor burden FL who received R-based chemotherapy.12 Another study demonstrated an association between high FDG avidity of FL and the tumor microenvironment13 ; therefore, we hypothesize that FDG uptake by tumor microenvironment cells may be contributing to the SUVmax in FL, which could alter the association between SUVmax and HT. Furthermore, because the earliest transformation was reported 6.8 months from randomization, it is unlikely that transformation was present at baseline.
Patients with FL who undergo HT have a poor prognosis, and there is a need for improved measures to identify those at risk. Multivariate analysis showed that ECOG PS of 2 and elevated LDH at baseline were prognostic of HT, which is consistent with the findings of a previous study in patients with FL.14 Also, although it may be expected that patients at risk for HT respond better to CHOP compared with bendamustine, our analysis did not identify any significant association between the chemotherapy regimen and HT.
The lack of predictive results for bSUVmax and bSUVrange reported in this analysis does not necessarily apply to PET findings at the time of relapse, when HT may be more frequent.
In conclusion, in this study, neither bSUVmax nor bSUVrange predicted HT in the GALLIUM study, suggesting that there may be little benefit in rebiopsy of lesions to exclude HT based on SUVmax alone before initiating therapy in de novo patients.
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available at https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. Further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents can be found at https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
Presented at the 7th International Workshop on PET in Lymphoma and Myeloma, Menton, France, 4-6 October 2018.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by F. Hoffmann-La Roche Ltd. Third-party medical writing assistance, under the direction of F.M., was provided by Helen Cathro and Danny Hawker (Gardiner-Caldwell Communications) and funded by F. Hoffmann-La Roche Ltd. S.F.B. acknowledges support from the National Institute for Health Research (RP-2-16-07-001). King’s College London and University College London Comprehensive Cancer Imaging Centre is funded by Cancer Research UK and the Engineering and Physical Sciences Research Council in association with the Medical Research Council and Department of Health (England).
The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health and Social Care (England).
Authorship
Contribution: D.S., F.M., J.T., and T.N. conceived and designed the study and collected and assembled the data; F.M., H.B., M.M., J.T., and S.F.B. analyzed and interpreted the data; and all authors wrote and had final approval of the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: F.M. is a former employee of Roche. S.F.B. has served on the board of directors or advisory committee for F. Hoffmann-La Roche and has received research funding from the National Institutes of Health Research, Cancer Research UK, Engineering and Physical Sciences Research Council, Medical Research Council, Department of Health (England), Siemens Medical, Bristol-Myers Squibb, Amgen, Celgene, and F. Hoffmann-La Roche. M.M. has received honoraria from F. Hoffmann-La Roche. H.B. is employed by PAREXEL (external business partner with Roche Products Ltd). T.N. and D.S. are employed by and have ownership interests in F. Hoffmann-La Roche. J.T. has received research funding from F. Hoffmann-La Roche, Janssen, Celgene, BeiGene, and Pharmacyclics, travel support from F. Hoffmann-La Roche, and is an unremunerated member of an advisory board for F. Hoffmann-La Roche, Janssen, Celgene, and Takeda.
Correspondence: Farheen Mir, Royal Marsden Hospital, Downs Rd, Sutton SM2 5PT, United Kingdom; e-mail: farheen.mir@nhs.net.