In this issue of Blood, Condoluci et al report that, using a large international cohort of 4933 patients from 11 groups, they have developed an international prognostic score (IPS-E) for predicting time to first treatment (TTFT) in patients with asymptomatic, early-stage CLL.1 Although there have been multiple past publications on prognostication in CLL, previous models have predominantly addressed overall survival as the end point of interest.2 Although survival is obviously of interest for both physicians and patients, the long survival duration of patients with early-stage CLL3 means that a more immediately relevant and often asked question is: “Doctor, how long do I have before I have to put my life on hold and accept treatment for my leukemia?”
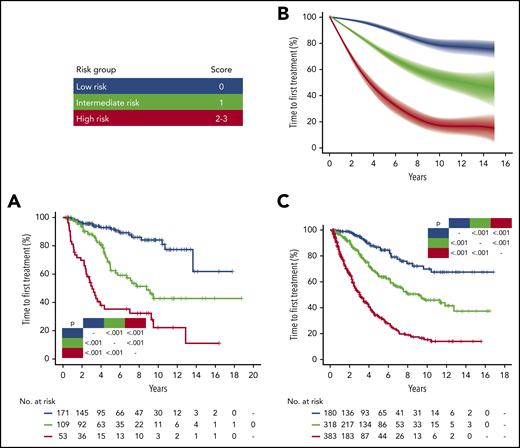
IPS-E stratified time to first treatment in early stage CLL patients managed with active surveillance. (A) Kaplan-Meier curves of TTFT stratified by IPS-E in the University of Eastern Piedmont discovery cohort. (B) Meta-analytic estimate of TTFT by IPS-E and the corresponding variability across the 9 Binet A validation cohorts. The bold line shows the cubic spline fitted on the meta-analytic estimate of the cumulative proportion of TTFT at each timepoint. The shadow shows the cubic splines fitted on the meta-analytic estimate of the 95% confidence interval of the cumulative proportion of TTFT at each timepoint. (C) Kaplan-Meier curves of time to first treatment stratified by IPS-E in the Mayo Clinic validation cohort. Blue, low-risk; green, intermediate-risk; red, high-risk by IPS-E. Multiplicity corrected P values by pairwise log-rank tests are shown. See Figure 2 in the article by Condoluci et al that begins on page 1859.
IPS-E stratified time to first treatment in early stage CLL patients managed with active surveillance. (A) Kaplan-Meier curves of TTFT stratified by IPS-E in the University of Eastern Piedmont discovery cohort. (B) Meta-analytic estimate of TTFT by IPS-E and the corresponding variability across the 9 Binet A validation cohorts. The bold line shows the cubic spline fitted on the meta-analytic estimate of the cumulative proportion of TTFT at each timepoint. The shadow shows the cubic splines fitted on the meta-analytic estimate of the 95% confidence interval of the cumulative proportion of TTFT at each timepoint. (C) Kaplan-Meier curves of time to first treatment stratified by IPS-E in the Mayo Clinic validation cohort. Blue, low-risk; green, intermediate-risk; red, high-risk by IPS-E. Multiplicity corrected P values by pairwise log-rank tests are shown. See Figure 2 in the article by Condoluci et al that begins on page 1859.
To answer this question, Condoluci et al examined features associated with TTFT in a training set of 333 consecutive patients followed over a median of 7.2 years at the University of Eastern Piedmont.1 This cohort was remarkably complete in terms of biological information, with immunoglobulin HV (IgHV) mutation, fluorescence in situ hybridization, and TP53 mutational status known in all patients. Using a multivariable model, 3 factors of equal weighting (1 point each) were identified to be independently predictive of TTFT: unmutated IgHV status, lymphocyte count higher than 15 × 109/L, and palpable nodes. Successful validation of these factors in 10 other European and US cohorts led to establishment of the IPS-E, which divided patients into approximately thirds: patients with score 0 (30%) have a low risk of requiring treatment (2.0 per 100 person-years), score 1 (36%) yields an intermediate risk of requiring treatment (6.1 per 100 person-years), and score 2 to 3 (34%) indicates a high risk of requiring treatment (16.1 per 100 person-years). Importantly, although TP53 deletions/mutations are key determinants of treatment response and overall survival after commencement of therapy, in this study, TP53 aberrations are not found to be independently predictive of TTFT in otherwise asymptomatic patients. This observation confirms previous reports that TP53 aberrant CLL can behave in an indolent manner when accompanied by favorable biological risk factors such as mutated IgHV,4,5 thus underscoring the importance of not managing early-stage patients differently solely on the basis of TP53 status.
Overall, the IPS-E is a simple tool that has immediate relevance to the clinician counseling newly diagnosed patients in the clinic. However, it does depend crucially on IgHV mutation testing, which is not considered an essential test at diagnosis by the current IWCLL guidelines,6 and there remain many areas of the world where IgHV mutation testing is not performed by community physicians practicing outside of academic centers. As an example, in the current study, comprising patients enrolled predominantly from specialist CLL research groups, IgHV status is known in all but 15 of the 4933 patients. In contrast, data from the real-world InformCLL registry showed that testing for IgHV mutation status occurred in only 11% of patients managed in the US community setting.7 In contrast, fluorescence in situ hybridization and sequencing tests for TP53 deletions and mutations are not required at diagnosis, but should be checked before therapy, as TP53 status significantly influences the choice of treatment.6,8
Finally, it is important to remember that outside of the clinical trial setting, treatment of CLL is not indicated until the development of symptomatic disease meeting published criteria.6,8 Phase 3 studies of FCR vs watch and wait9 and ibrutinib vs placebo10 have yet to show overall survival benefit for early intervention in any risk group. The IPS-E is a shiny new tool for risk stratifying patients in the clinic; however, good clinical judgement and adherence to clinical guidelines remain the cornerstones of modern CLL management.
Conflict-of-interest disclosure: C.S.T. reports receiving research funding from Janssen and AbbVie and honoraria from Janssen, Pharmacyclics, and AbbVie. J.F.S. reports receiving research funding from AbbVie and Janssen and honoraria from AbbVie, Celgene, Genentech, Janssen, Roche, Takeda, and Sunesis.