In this issue of Blood, Oberbeck et al1 have undertaken a comprehensive evaluation of the biology and genetics of T-cell prolymphocytic leukemia (T-PLL), focusing on the cooperation between the T-cell receptor (TCR) and the protooncogene T-cell leukemia 1A(TCL1A) in promoting the growth and survival of T-PLL cells.
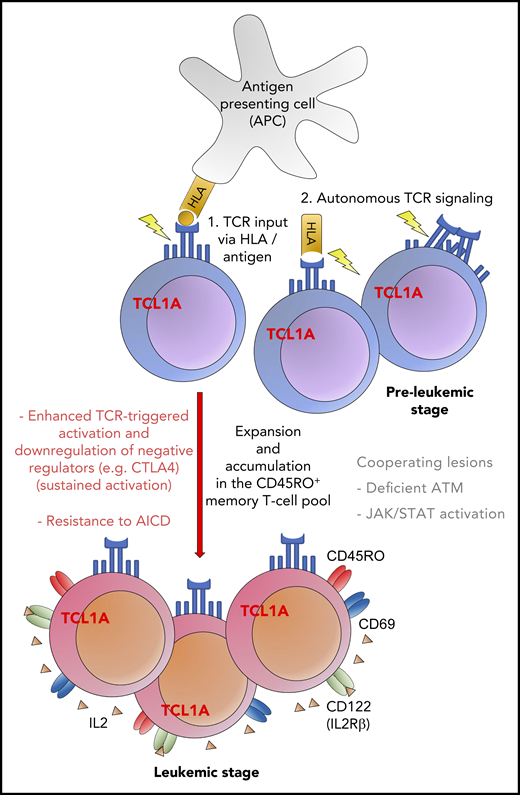
Proposed model of the proleukemogenic cooperation of TCL1A with TCR signaling. TCL1A confers a survival benefit, supported by the effect of additional genomic alterations (eg, ATM) and contributes to resistance to programmed cell death (AICD). The figure has been adapted from Figure 7E in the article by Oberbeck et al that begins on page 2786.
Proposed model of the proleukemogenic cooperation of TCL1A with TCR signaling. TCL1A confers a survival benefit, supported by the effect of additional genomic alterations (eg, ATM) and contributes to resistance to programmed cell death (AICD). The figure has been adapted from Figure 7E in the article by Oberbeck et al that begins on page 2786.
T-PLL is a rare, aggressive, and ultimately fatal malignancy. To date, understanding of the pathogenesis of T-PLL has been elusive, and successful therapy even more so. In this paper, Oberbeck et al report the results of a series of elegant experiments on a large collection of 188 primary T-PLL cells, supplemented by murine models to validate their findings. First, the authors confirm that surface marker and gene expression reveal a central memory T-cell phenotype in the majority of T-PLL samples, albeit with some heterogeneity, which is distinct from normal naive T cells. Second, they show that the T-PLL cells have an activated phenotype, with overexpression of proliferation markers, such as CD38, CD69, CD40L, and Ki67. Interestingly, this does not appear to change during disease progression, although further longitudinal studies may be required to confirm this. This activation state is maintained by constant low-level antigen stimulation via the TCR and is coupled to downregulation of inhibitory coreceptors (eg, CTLA4), resulting in persistence of the T-PLL clone. Resting memory T cells depend on low-level TCR input, but the threshold for activation is much lower in T-PLL than in normal T cells. Third, Oberbeck et al interrogated the role of TCL1A, which has been recognized for >20 years as the most frequently occurring genetic alteration in T-PLL.2 This antiapoptotic protooncogene has a twofold impact (see figure). It enhances the response to T-cell stimulation leading to a growth advantage over normal T cells and facilitates transformation. In addition, it promotes resistance to activation-induced programmed cell death (AICD) via its interaction with the prosurvival kinase AKT. The fact that most T-PLL cells are ataxia telangiectasia mutated (ATM) deficient further compromises the intracellular apoptotic machinery.3 All of these features, memory T-cell phenotype, activation markers, and high expression of TCL1 protein, are associated with poor clinical outcome when queried in the patient cohort. For example, overall survival was 99 months for T-PLL patients with low (<5%) expression of TCL1A compared with 21 months for those patients with high expression (>5%).1 Indeed, promotion of growth and failure of apoptosis are the hallmarks of successful leukemia. Furthermore, the long-term persistence of apoptosis-resistant memory T cells renders them subject to additional transformational events, such as JAK/STAT pathway aberrations, which may play a role in disease progression.
This picture of chronic receptor stimulation, enhanced intracellular signaling, proliferation, and prolonged survival is similar to other lymphoid leukemias, such as chronic lymphocytic leukemia (CLL) and T-cell large granular lymphocyte leukemia. From the translational viewpoint, it makes sense to target these signaling pathways therapeutically. The use of agents that block the B-cell receptor signaling pathway and/or remove the resistance to apoptosis has been a very successful strategy in CLL.4 It is of interest, therefore, that anecdotal clinical experience reported here and supported by some in vitro drug sensitivity assays,5 suggests that a similar approach may be effective in T-PLL. The mechanisms involved have not yet been fully elucidated. A single patient with refractory T-PLL responded to the combination of ibrutinib (BTK/ITK inhibitor) and venetoclax (BCL2 inhibitor).1 This potential treatment strategy will need confirmation within a clinical trial but may provide a platform for a novel therapeutic approach, combining blockade of TCR- and survival-signaling pathways. The recent standardization of criteria for diagnosis and treatment response evaluation by the T-PLL International Study Group will facilitate such clinical trial activity.6 Future work also needs to integrate the pathogenetic model proposed in this paper with the other aberrant pathways identified in T-PLL, including genomic lesions in ATM and JAK/STAT signaling.
This paper is an important contribution to the field of T-PLL, a very rare disease with limited treatment options and a hitherto dismal outcome for patients, offering some hope of a new way forward.
Conflict-of-interest disclosure: The author declares no competing financial interests.