Abstract
Numerous studies have reported significant associations between ABO blood group and risk of cardiovascular disease. These studies have consistently demonstrated that thrombotic risk is significantly reduced in individuals in blood group O. Nevertheless, the biological mechanisms through which ABO influences hemostasis have remained poorly understood. Exciting recent data have provided novel insights into how these ABO effects are modulated and have highlighted that ABO group significantly influences platelet plug formation at sites of vascular injury (primary hemostasis). In particular, ABO affects multiple aspects of von Willebrand factor (VWF) biology. In keeping with their reduced thrombotic risk, plasma VWF levels are ∼25% lower in healthy group O compared with healthy group non-O individuals. In addition, blood group O VWF demonstrates enhanced susceptibility to ADAMTS13 proteolysis. Finally, preliminary findings suggest that the interaction of group O VWF with platelets may also be reduced. Although the molecular mechanisms underlying these ABO effects on VWF have not been fully elucidated, it seems likely that they are mediated in large part by the ABO(H) carbohydrate structures that are carried on both the N- and O-linked glycans of VWF. Interestingly, ABO(H) determinants are also expressed on several different platelet surface glycoprotein receptors. Recent studies support the hypothesis that ABO group not only exerts major quantitative and qualitative effects on VWF, but also affect specific aspects of platelet function. Given the severe morbidity and the mortality associated with thrombotic disorders, defining the mechanisms underlying these ABO effects is not only of scientific interest, but also of direct clinical importance.
Introduction
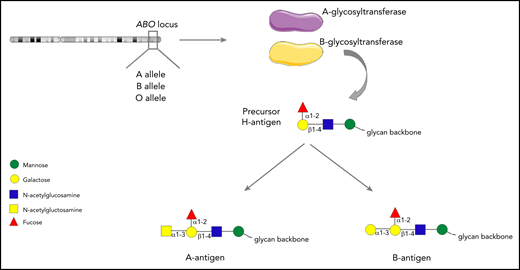
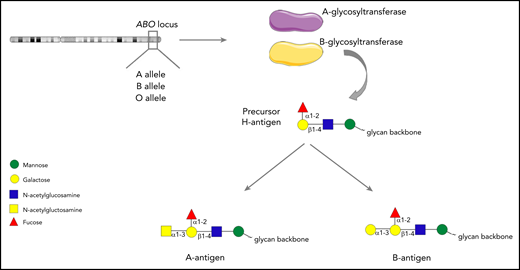
The ABO blood group system was first described by Karl Landsteiner in 1900. Although traditionally regarded as pertaining to red blood cells, the carbohydrate structures that comprise the ABO blood group system (A, B and H determinants) are expressed on a range of different cell types, including platelets and endothelial cells (ECs).1-3 ABH antigens are terminal sugar structures at the end of complex glycan chains.4 In brief, A and B alleles at the ABO locus on chromosome 9 encode either A- or B-glycosyltransferase enzymes.5 These transferases catalyze the addition of specific sugar residues so that precursor core H glycan structures are converted to form either A antigen (GalNAc α1→3 [Fuc α1→2] Galβ 1→4 GlcNAc β1→), or B antigen (Gal α1-3 [Fuc α1-2] Galβ 1-4 GlcNAc β1-; Figure 1). Consequently, A and B structures differ only with respect to a single terminal sugar moiety (N-acetylgalactosamine vs d-galactose). Group O individuals lack any A- or B-transferase activity and consequently continue to express the basic H glycan structure (Fuc α1-2] Galβ 1-4 GlcNAc β1-) at the termini of their oligosaccharide chains.4
The ABO histo-blood group system. A or B alleles at the ABO locus on chromosome 9 encode glycosyltransferase enzymes. These enzymes convert precursor H carbohydrate structures into A or B antigenic determinants, which differ only in a single terminal sugar residue.
The ABO histo-blood group system. A or B alleles at the ABO locus on chromosome 9 encode glycosyltransferase enzymes. These enzymes convert precursor H carbohydrate structures into A or B antigenic determinants, which differ only in a single terminal sugar residue.
The central importance of ABO blood group in blood transfusion and transplantation practice is well established. Interestingly, significant associations between ABO blood groups and a variety of other diseases have also been described.1 These include duodenal ulceration, gastric carcinoma, and several different types of sepsis (eg, Helicobacter pylori, Salmonella typhi, and Plasmodium falciparum).6,7 Recent studies have shown that ABO blood group also influences susceptibility to severe COVID-19 infection.8,9 Numerous studies have reported that ABO also constitutes an important independent risk factor for cardiovascular disease and venous thromboembolism.6,10 In addition genome-wide association studies have demonstrated that ABO blood group is not only a risk factor for atherogenesis, but is also important in the pathogenesis of acute coronary syndrome and myocardial infarction.11,12 Moreover, clinical studies have demonstrated that infarct size and overall mortality are significantly reduced in patients of blood group O.13 Despite these data, the biological mechanism(s) through which ABO type determines thrombotic risk remains poorly understood. A relationship between ABO blood group and blood coagulation was first proposed more than 60 years ago.14 In this article, we review accumulating recent data that have provided novel insights into how ABO type directly influences primary hemostasis. In particular, we consider the multifaceted ways through which ABO blood group influences the lifecycle of the key coagulation glycoprotein von Willebrand factor (VWF).
VWF glycosylation
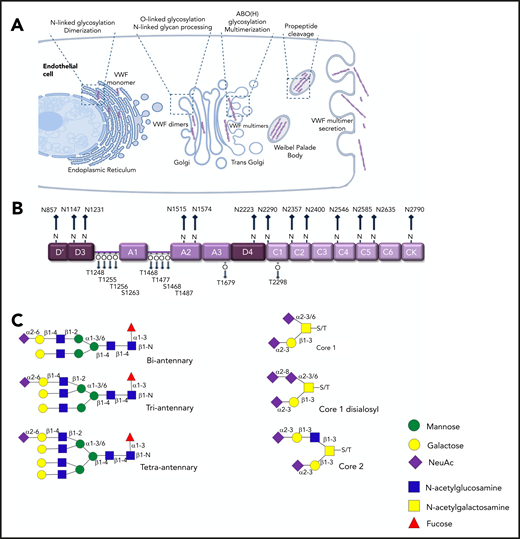
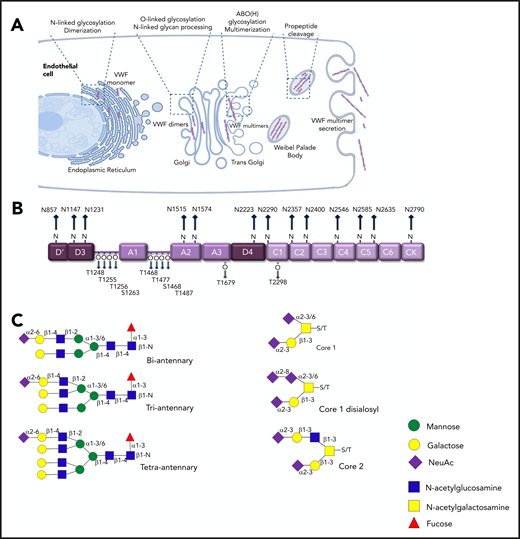
Under normal conditions, in vivo biosynthesis of VWF is restricted to ECs and megakaryocytes only.15,16 This biosynthesis includes complex posttranslational modification that is completed before VWF is secreted into the plasma (Figure 2A). In the endoplasmic reticulum (ER), VWF monomers are assembled into dimers through formation of C-terminal disulphide bonds. Subsequently, in the Golgi, VWF dimers are assembled into high-molecular-weight multimers (HMWMs) after another round of N-terminal disulphide bond formation. Consequently, plasma VWF exists as a series of heterogeneous oligomers.16 The posttranslational modification of VWF also includes significant glycosylation.17 This glycosylation has direct clinical relevance because the oligosaccharide structures expressed on VWF regulate multiple aspects of its biological activity.16,18 Glycosylation begins in the ER, where high-mannose oligosaccharides are added to specific asparagine residues (N-linked sites) in VWF monomers before dimerization. As VWF passes through the Golgi, these N-glycans are modified to generate complex branching carbohydrate structures. Concurrently, O-linked glycosylation takes place, which involves the addition of more simple glycan structures to specific serine and threonine residues in VWF. As a result, VWF monomer contains 13 N-linked and 10 O-linked glycans located at specific sites distributed across the mature monomer (Figure 2B), with oligosaccharide accounting for almost 20% of the final monomeric mass.
N- and O-linked glycosylation of human VWF. (A) Before secretion from ECs, VWF undergoes complex posttranslational modification that includes significant N- and O-linked glycosylation. (B) Each VWF monomer contains 13 N- and 10 O-linked glycan chains. (C) Complex biantennary, triantennary, and tetra-antennary chains are expressed on the N-glycans of VWF. In contrast, core 1, core 2, and disialosyl structures are common on the O-glycans.
N- and O-linked glycosylation of human VWF. (A) Before secretion from ECs, VWF undergoes complex posttranslational modification that includes significant N- and O-linked glycosylation. (B) Each VWF monomer contains 13 N- and 10 O-linked glycan chains. (C) Complex biantennary, triantennary, and tetra-antennary chains are expressed on the N-glycans of VWF. In contrast, core 1, core 2, and disialosyl structures are common on the O-glycans.
An initial study of VWF glycan structures, in which western blot and sequential glycosidase digestion were used, reported that sialylated biantennary chains constitutes ∼80% of all the N-linked glycans on VWF.19 In contrast, a disialylated core-1 tetra-saccharide structure (known as the T antigen) accounts for 70% of the total O-glycan population.20 More recently, mass spectrometry (MS) studies have been performed on pooled human plasma–derived concentrates to definitively map the N- and O-glycomes of VWF (Figure 2C).21-24 These MS data have uncovered marked heterogeneity among VWF carbohydrate structures, which may be attributable in part to the pooled VWF used in these studies. More than 100 distinct N-glycan compositions have already been characterized.21 Importantly, the MS studies have further shown that the predominant type of glycan structures expressed at different sites on VWF varies significantly. For example, complex tetra-antennary chains are more common at N1515 and N1574 in the VWF A2 domain. Conversely, simpler biantennary chains predominate at the N-glycan sites in the D3 domain (N1147) and D4 domains (N2223 and N2290).21,24
Unlike the N-glycans that are distributed across the VWF monomer, 8 of the 10 O- glycans (T1248, T1255, T1256, S1263, T1468, T1477, S1486, and T1487) are clustered in 2 groups on either side of the A1 domain (Figure 2B). In addition, the O-glycans of VWF typically exist as short mucin-type structures.22,23 Nevertheless, recent MS studies have again demonstrated significant heterogeneity between these O-linked carbohydrates.23 Eighteen different glycan structures have been described including both core 1 and core 2 structures. Core 1 structures are generally small glycans in which Galβ1-3GalNAc is linked to the serine or threonine residue. In contrast, core 2 structures possess an additional branching N-acetylglucosamine attached to core 1 glycan (Figure 2B). Different types of carbohydrate chains have been shown to be preferentially expressed at specific O-linked sites on VWF. For example, core 2 glycan structures were more common on the cluster of O-glycans located at the C-terminal end of the A1 domain (T1468, T1477, S1486, and T1487).22,23 Cumulatively, these new insights into VWF glycosylation highlight that, even in healthy individuals, plasma VWF is likely to circulate in many different site-specific glycoforms.
ABO(H) blood group structures on VWF glycans
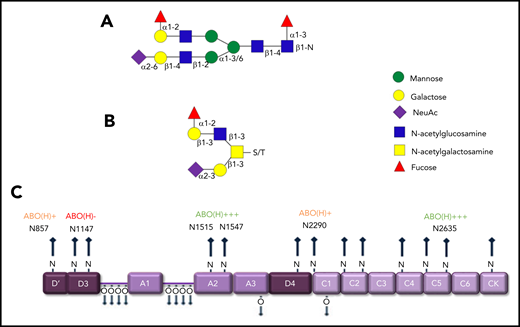
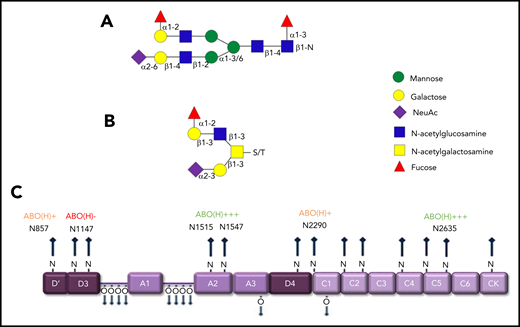
Previous studies have shown that covalently linked ABO(H) blood group determinants are expressed as terminal residues on both the N- and O-glycans of humans VWF (Figure 3A-B).6,25 Overall, ∼15% of VWF N-glycans and 1% of O-glycans were shown to carry ABO(H) structures.21,22 Besides VWF, factor VIII (FVIII), and α2-macroglobulin, few other circulating glycoproteins carry ABO(H) structures. MS analyses have demonstrated that ABO(H) expression is not restricted to particular VWF domains. Rather, it is distributed across all the N-glycosylation sites, with the exception of N1147.21 Highest levels of N-linked ABO(H) expression have been observed at N1515, N1574, and N2365 (Figure 3C). In contrast, N857, N2290, and N2585 represented poorly fucosylated sites containing only 1% to 3% ABO(H) structures.21 Although the specific glycosyltransferase enzymes required to synthesize ABO(H) structures are present in normal plasma, analyses performed in patients after ABO-mismatched bone marrow transplantation suggest that ABO(H) determinants are added to VWF before its secretion from ECs.26
ABO blood antigens are present on plasma-derived VWF. (A-B) Covalently linked ABH antigens are carried as terminal sugar moieties on the termini of both N- and O- glycans of human plasma-derived VWF. (C) Quantitative expression of ABO(H) determinants varies between specific N-linked sites on the VWF monomer.
ABO blood antigens are present on plasma-derived VWF. (A-B) Covalently linked ABH antigens are carried as terminal sugar moieties on the termini of both N- and O- glycans of human plasma-derived VWF. (C) Quantitative expression of ABO(H) determinants varies between specific N-linked sites on the VWF monomer.
Quantitative ABO(H) expression levels on plasma VWF is regulated by several factors. First, A and B antigen loading on VWF is influenced by ABO genotype, with significantly reduced expression in heterozygous (AO or BO) compared with homozygous individuals (AA or BB).27,28 Second, there is significant heterogeneity in the amount of ABO(H) carried on VWF synthesized by EC located in different vascular beds.26 Third, A and B determinant loading is significantly higher on VWF secreted immediately after administration of desmopressin (DDAVP).29 Desmopressin triggers secretion of VWF stored in Weibel-Palade bodies within ECs, and is widely used in the treatment of von Willebrand disease (VWD). Finally, for reasons that have not been defined, A and B determinants are not expressed on VWF stored within platelet α-granules.30,31 This observation is intriguing, given that covalently linked ABO(H) blood group determinants are carried on several platelet membrane glycoproteins (including GPIb, IIb, and IIIa).30 Because A and B antigens are expressed on these other platelet glycoproteins, it is clear that megakaryocytes possess all the necessary glycosyltransferases for blood group antigen assembly. However, the posttranslational modification pathway of VWF within megakaryocytes clearly differs from that in EC, such that VWF glycans are not influenced by these A and B glycosyltransferases.
ABO blood group influences plasma VWF:Ag levels
Plasma VWF antigen (VWF:Ag) levels vary over a wide range in the healthy population (normal range, ∼50-200 IU/dL).32,33 This variation has direct clinical relevance. Reduced VWF:Ag levels are responsible for the commonest inherited bleeding disorder VWD.32,34 Conversely, elevated plasma VWF:Ag levels (>150 IU/dL) constitute a dose-dependent risk factor for thrombosis.35 Studies in twins have reported that 66% of the total variation in plasma VWF:Ag levels is genetically determined.36 Moreover, 30% of this genetic influence on VWF levels is attributable to an effect of ABO blood group.36
A major effect of ABO on plasma VWF:Ag levels was first reported by Preston and Barr in 196437 and has been confirmed by multiple independent groups in studies involving a variety of different ethnicities.25,38 From the results of these studies, it is clear that healthy group O individuals have plasma VWF:Ag levels that are ∼20% to 30% lower than those of non-O individuals. Although some variation has been reported among the non-O groups across different studies, group AB individuals tend to have the highest plasma VWF:Ag levels. ABO blood group has similar effects on plasma FVIII levels, although these effects appear to be predominantly determined through the changes in VWF levels. The magnitude of the effect of ABO on plasma VWF:Ag levels is striking, given that ABO(H) glycan determinants differ only with respect to a single terminal sugar moiety and are expressed on only a minority of the N- and O-linked glycans of VWF.21,22
In addition to the association between ABO phenotype and VWF levels, recent studies have demonstrated that genotype at the ABO locus also has a quantitative effect on VWF.27,28,39 Thus, heterozygous individuals carrying an O-allele (genotypes AO or BO) had significantly reduced VWF:Ag levels compared with subjects with genotypes AA, BB, or AB.27,28,39 Furthermore, plasma VWF:Ag levels were significantly reduced in individuals with the rare Bombay blood group phenotype (in which H antigens are not expressed) compared with group O controls.40 Of note, several reports have described a weak association between the secretor blood group locus and plasma VWF:Ag levels.41,42 This finding is interesting, as the secretor blood group is related to ABO, in that it is also defined by the presence or absence of specific terminal sugar moieties on carbohydrate chains. Interestingly, however, in keeping with the observation that platelet VWF does not carry covalently linked ABO(H) determinants, platelet-VWF levels are not influenced by ABO group.30
The relationship between ABO and plasma VWF:Ag levels has important clinical implications with respect to the diagnosis of quantitative VWD where group O individuals are significantly overrepresented.38 This group O predominance is particularly evident in patients registered with a diagnosis of low VWF levels (range, 30-50 IU/dL).43 Collectively, these data have led to the suggestion that ABO-specific reference ranges should be considered for VWD diagnosis.33 Critically, despite this translational relevance, the biological mechanism(s) through which ABO regulates VWF levels has not been clearly defined. Nevertheless, it is clear that the dosage effect of the ABO locus in determining ABO(H) determinant loading on VWF glycans parallels its effect on plasma VWF:Ag levels.27,28
Besides the effects of ABO blood group in regulating plasma VWF:Ag levels, ethnicity has been demonstrated to have an influence, with significantly lower levels in white compared with African Americansindidividuals.44 This effect of race has been estimated to account for 7% of the total variance in VWF levels. The prevalence of ABO blood group phenotypes also varies between different racial and ethnic groups. For example, in the United States, the prevalence of group O was higher in one study in Hispanic (56.5%), North American Indian (54.6%), and black non-Hispanic (50.2%) compared with white non-Hispanic (45.2%) individuals.45 Importantly however, despite interethnic variations in ABO distribution, linear regression analysis has demonstrated that the effect of race on VWF:Ag is independent of the ABO effect.44
ABO blood group and VWF functional activity
For many years, VWF ristocetin cofactor activity (VWF:RCo assay) has been used to assess VWF function.46 In this assay, ristocetin binds to the VWF A1 domain and enables VWF to interact with platelet Gp1b under static conditions, thereby triggering platelet agglutination. Despite its widespread use, the VWF:RCo assay demonstrates significant inter- and intralaboratory coefficients of variation (CVs).47 Previous studies have demonstrated that modifications in VWF glycan structures significantly influence its biological activity (Figure 4).18 In particular, specific roles for ABO(H) blood group determinants in regulating VWF-platelet interaction have been proposed. Sarode et al purified ABO-specific VWF from fresh frozen plasma.48 Removal of A antigenic determinants from group A VWF after digestion with α-N-acetylgalactosaminidase (Azyme) was associated with a significant reduction (>30%) in VWF:RCo activity. Treatment of group B VWF with α-galactosidase (Bzyme) had a similar effect. Conversely, these digestions did not cause any reduction in VWF collagen binding, suggesting that VWF multimer distribution was not affected. Moreover, control experiments demonstrated that treatment of group A VWF with Bzyme or group B VWF with Azyme is not associated with any reductions in VWF:RCo. Based upon these findings, the researchers postulated that AB antigen expression may play a role in rendering VWF less compact and thus more capable of platelet interaction.48 Although the clinical significance of these data remains unclear, several studies performed on healthy controls have reported differential effects of ABO blood group on VWF functional assays (including VWF:RCo and VWF:collagen binding).44,49,50 Conflicting conclusions have been reported from some of these studies that may relate in part to the different types of assay conditions used, together with differences in ethnic origins. A recent study has defined polymorphisms in the VWF A1 domain (in particular D1472H) that attenuate the ability of VWF to bind ristocetin.51 As a result, these polymorphisms are associated with reduced VWF:RCo laboratory results, but not with any significant reductions in plasma VWF functional activity. Importantly, D1472H is common in the general population (particularly in African American individuals)51 and thus may have confounded the results of some of the previous cohort studies that sought to investigate whether the expression of ABO(H) on VWF has any functional effects.
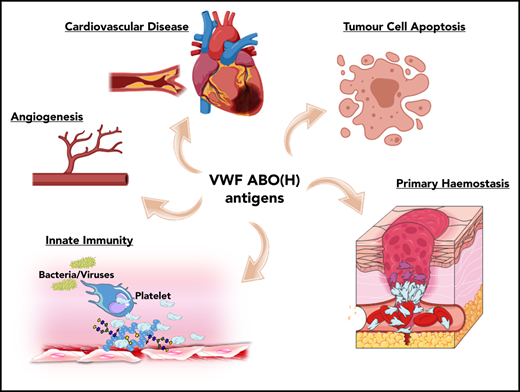
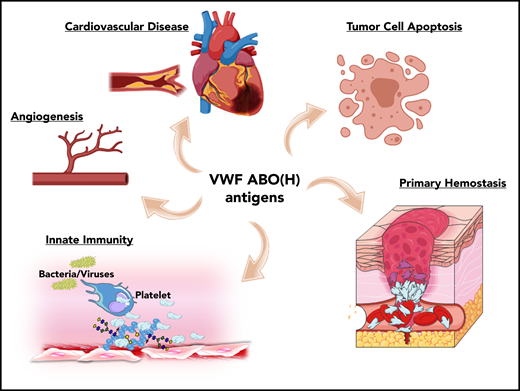
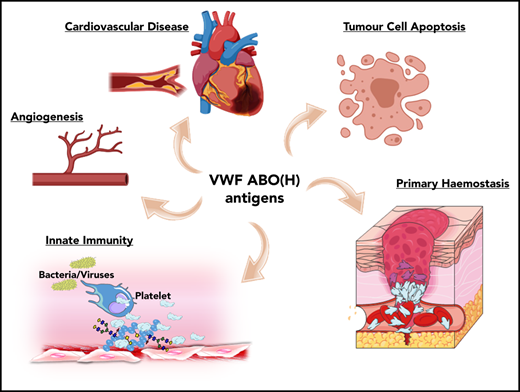
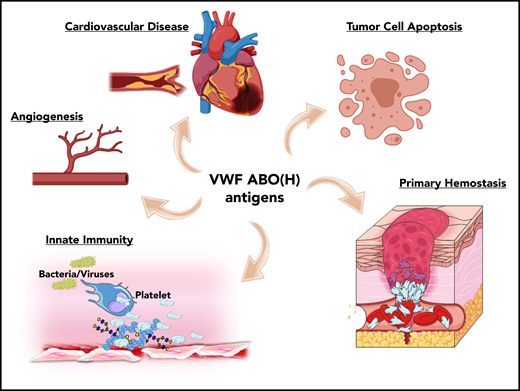
ABO blood group influences VWF functional biology. ABO blood group has a significant quantitative effect on plasma VWF:Ag levels and thus on the risk of cardiovascular disease. In addition, ABO may also influence a wide variety of other VWF functional properties (eg, on angiogenesis, wound healing, and innate immunity).
ABO blood group influences VWF functional biology. ABO blood group has a significant quantitative effect on plasma VWF:Ag levels and thus on the risk of cardiovascular disease. In addition, ABO may also influence a wide variety of other VWF functional properties (eg, on angiogenesis, wound healing, and innate immunity).
Besides its established role in maintaining normal hemostasis, exciting recent studies have identified a series of additional novel biological roles for VWF. For example, VWF has been shown to play important roles in inhibiting angiogenesis,52 promoting wound healing,53 and enhancing tumor cell apoptosis.54 Furthermore, accumulating evidence suggests that VWF plays important roles in regulating different aspects of innate immune responses and sepsis.55-57 Given data from a previous association study implicating ABO blood group in the pathobiology underlying various diseases,1 it would be intriguing to investigate whether these newly identified roles of VWF are relevant in this context. In addition, further studies are needed to specifically investigate how VWF glycans (including ABO(H) expression) affect these emerging novel biological functions.
ABO blood group and VWF proteolysis
In plasma, VWF circulates as a series of heterogeneous multimeric forms.16 Multimer distribution should be tightly regulated, as it is a critical determinant of VWF activity. HMWMs bind to both collagen and platelet GpIbα with enhanced affinity. Under normal conditions, VWF multimer distribution is regulated by ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type-1 repeats), which cleaves at a specific Tyr1605-Met1606 bond within the VWF A2 domain.58 The physiological importance of regulating VWF HMWM distribution is emphasized by the fact that ADAMTS13 deficiency is associated with circulating, pathological, ultralarge VWF multimers and thrombotic microvascular occlusion in thrombotic thrombocytopenic purpura (TTP).58,59 In contrast, loss of normal HMWM VWF caused by enhanced proteolysis is associated with a significant bleeding phenotype in type 2A VWD.32,34
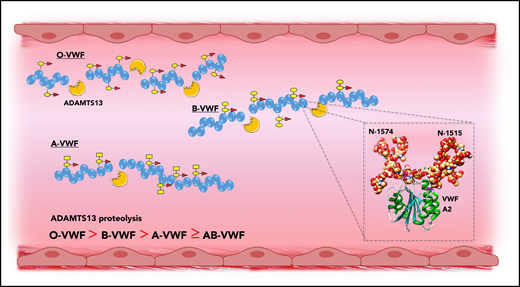
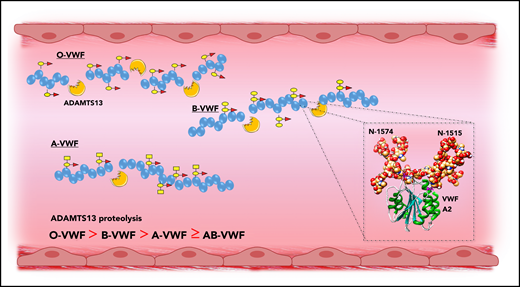
Previous studies have demonstrated that carbohydrate structures play an important role in regulating the susceptibility of VWF to proteolysis by ADAMTS13, as well as several other proteases including plasmin.31,60,61 More recently, accumulating evidence has shown that ABO(H) determinants on human VWF are of particular importance in this context. Bowen was the first to report that group O VWF was cleaved more rapidly by ADAMTS13 compared with non-O VWF (Figure 5).62 Differences in susceptibility to ADAMTS13 were also observed among the other non-O groups in the order B > A ≥ AB. A subsequent study confirmed this initial observation and reported that ADAMTS13 proteolysis is significantly enhanced in individuals with the rare Bombay phenotype, in whom ABO(H) determinants are not expressed.40 A role for terminal α2-6–linked sialylation on the N-glycans of VWF in modulating ADAMTS13 proteolysis has also been described.63 To date, the mechanisms through which ABO(H) and other carbohydrate structures on VWF serve to modulate susceptibility to ADAMTS13 have not been elucidated.62,64 Interestingly however, 2 conserved N-linked glycan sites (N1515 and N1574) are located in the VWF A2 domain adjacent to the ADAMTS13 cleavage site (Figure 5, inset).65 A site-directed mutagenesis study suggested a specific role for the glycans at N1574 in protecting VWF against ADAMTS13 proteolysis.61 In addition, results of a recent differential scanning fluorimetry study shown that N1574 glycosylation plays a role in stabilization of the A2 domain against unfolding.66 Cumulatively, these data are interesting, given that ABO(H) blood group determinants are strongly expressed as terminal sugar moieties on the complex N1574 glycan chain.21
ABO blood group modulates VWF proteolysis by ADAMTS13. ABO blood group regulates the susceptibility of VWF to proteolysis by ADAMTS13, with group O VWF being cleaved significantly more rapidly compared with non-O VWF. Although the mechanism underlying the effect of ABO group on ADAMTS13 susceptibility has not been defined, 2 N-linked glycan sites are located in the A2 domain adjacent to the ADAMTS13 cleavage site (inset).
ABO blood group modulates VWF proteolysis by ADAMTS13. ABO blood group regulates the susceptibility of VWF to proteolysis by ADAMTS13, with group O VWF being cleaved significantly more rapidly compared with non-O VWF. Although the mechanism underlying the effect of ABO group on ADAMTS13 susceptibility has not been defined, 2 N-linked glycan sites are located in the A2 domain adjacent to the ADAMTS13 cleavage site (inset).
That group O VWF demonstrates enhanced susceptibility to ADAMTS13 proteolysis has led to the suggestion that individuals with blood group O may be partially protected against TTP. The reduced plasma VWF:Ag levels associated with group O may also be important in this setting. This hypothesis has been investigated in 3 studies.67-69 Of those, 2 small retrospective studies reported a lower-than-expected distribution of group O among their TTP cohort, although the result failed to achieve statistical significance.67,69 However, in the third, larger, prospective study, 301 consecutive patients with TTP were enrolled and results were conflicting, with the conclusion that group O has no protective effect.68 Rather, Terrell et al found that blood group O represents an independent risk factor for TTP that is associated with severe ADAMTS13 deficiency.68 Although this paradoxical ABO effect was shown to be independent of race and ethnicity, the pathological mechanisms underpinning the association remain to be defined.
ABO blood group and VWF clearance
The mechanism(s) through which ABO blood group influences plasma VWF:Ag levels has not been clearly defined. Theoretically, this quantitative effect could be attributable to ABO-related differences in VWF biosynthesis within ECs. Alternatively, ABO may affect the rate of VWF plasma clearance in vivo. In vitro studies to directly investigate whether ABO influences VWF biosynthesis have proved challenging, because the glycan structures on VWF expressed in vitro differ from those on human plasma VWF.70,71 Nonetheless, current evidence suggests that ABO blood group does not significantly affect the rate of VWF synthesis or secretion in ECs. Studies have demonstrated that (1) ABO blood group has no effect on plasma VWF propeptide (VWFpp) levels72 ; (2) ABO does not influence platelet VWF:Ag levels30 ; and (3) ABO has no significant effect on the amount of VWF secreted after administration of desmopressin.29 In contrast, accumulating recent data support the hypothesis that the effect of ABO(H) blood group on plasma VWF:Ag levels is primarily due to differences in the VWF clearance rates of the blood groups. For example, Gallinaro et al showed that the half-life of VWF secreted after administration of desmopressin was affected by ABO blood group.73 In particular, VWF half-life was significantly shorter in individuals with group O compared with that in non-O individuals (10.0 vs 25.5 hours, respectively). In keeping with these findings, the VWFpp:VWFAg ratio (a surrogate marker for endogenous clearance rates) is also significantly elevated in group O subjects.74 Finally, a study showed that the half-life of infused FVIII is significantly shorter in patients in blood group O than in patients in the non-O group who have severe hemophilia A.75 Because most of the infused FVIII in these patients is cleared in complex with VWF, the ABO effect is most likely related to differences in the clearance rates of their endogenous VWF.
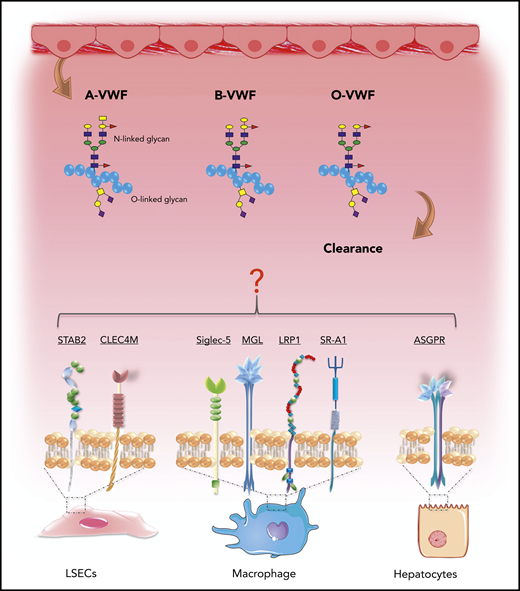
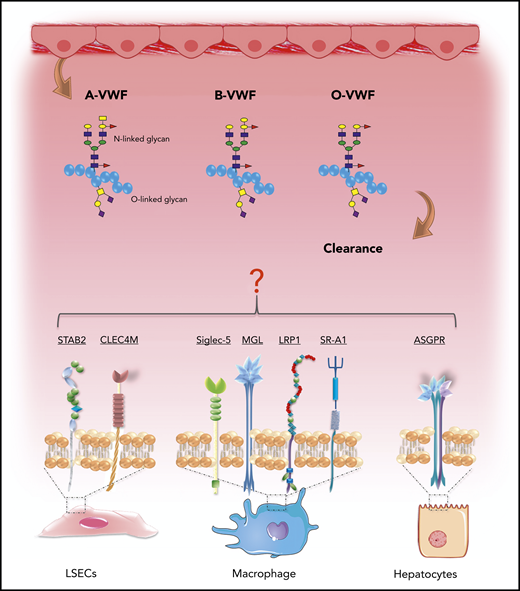
Recent studies have provided significant novel insights into the pathways involved in VWF clearance.76 Lenting et al showed that radio-labeled VWF is primarily targeted to the liver, and that VWF colocalizes with CD68+ Kupffer macrophage cells.77,78 Subsequent in vitro studies confirmed that macrophages bind VWF in a dose-dependent manner and that macrophage binding is followed by VWF uptake and degradation (Figure 6).65,78,79 More recent studies have suggested that liver sinusoidal ECs also contribute to VWF clearance.80 Furthermore, enhanced VWF clearance has been shown to play a key role in the pathogenesis of VWD.76,81 Notwithstanding these data, the biological mechanisms underpinning the enhanced clearance of group O VWF remain unknown. Importantly, studies have demonstrated that VWF clearance is not influenced by VWF multimer size and is thus independent of ADAMTS13 proteolysis.77,82 Consequently, the enhanced susceptibility to ADAMTS13 proteolysis and increased clearance observed in group O individuals appear to be independent phenomena. In this context, it is interesting that Groeneveld et al recently reported that ABO had so significant effect on the clearance of human VWF in VWF−/− mice.83 Critically, however, mice do not express A and B determinants.84 Moreover, human VWF clearance in mice is markedly enhanced compared with that of endogenous murine VWF or compared with the normal half-life of VWF in humans.77,83 In addition, some of the receptors implicated in regulating human VWF clearance (including CLEC4M and Siglec-5) are not expressed in mice.76
ABO blood group influences VWF clearance in vivo. ABO blood group has a significant effect on the plasma half-life of human VWF. Blood group O is cleared significantly more rapidly than group A or B VWF. Although the molecular mechanism responsible for this enhanced clearance remains unknown, recent studies have identified a series of putative VWF clearance receptors.
ABO blood group influences VWF clearance in vivo. ABO blood group has a significant effect on the plasma half-life of human VWF. Blood group O is cleared significantly more rapidly than group A or B VWF. Although the molecular mechanism responsible for this enhanced clearance remains unknown, recent studies have identified a series of putative VWF clearance receptors.
Several C-type lectin receptors have been implicated in regulating VWF clearance, including asialoglycoprotein,85 the macrophage galactose-type lectin,86 and CLEC4M (Figure 6).87 The extracellular regions of the lectin receptors all contain carbohydrate recognition domains that interact with specific carbohydrate determinants on glycoproteins. Although VWF sialylation is critical in protecting VWF against clearance,88 to date, there is no evidence that ABO(H) carbohydrates influence binding to any of these lectin receptors. In addition, several scavenger receptors have also been shown to bind VWF, including low-density lipoprotein receptor-related protein-1 (LRP1),65,89 the scavenger receptor class A member I (SR-A1)90 and stabilin-2 (STAB2) (Figure 6).80 Recent studies have highlighted that glycan structures at N1515 and N1574 in the A2 domain influence VWF half-life by protecting against clearance through the macrophage LRP1 scavenger receptor.65,91 Further studies are needed, to determine whether N-linked ABO(H) determinants on VWF influence physiological in vivo clearance via this pathway.
ABO blood group and platelet function
Although ABO blood group determinants are not carried on the N-glycans of platelet-VWF, studies have shown that they are expressed on the surface of several platelet membrane glycoproteins and glycolipids.92 Among the glycoproteins that express ABH antigens are GPIb, IIa, IIIa, IV, and V, as well as platelet EC adhesion molecule-1 (PECAM-1).30,93-95 At sites of vascular injury, VWF plays a key role in facilitating platelet plug formation by acting as a bridge between the exposed subendothelial collagen and specific receptors expressed on the surface of platelets.34 Once VWF becomes tethered under shear stress conditions, it interacts with GPIb-IX-V and GPIIb-IIIa complexes on platelet surfaces. The observation that ABO(H) glycans are also expressed on these surface receptors has led to the hypothesis that ABO blood group may impact primary hemostasis by influencing platelet function.92 This hypothesis is supported by several recent lines of evidence.
First, as part of the Genetic Analysis of Idiopathic Thrombophilia (GAIT-2) project, a genome-wide association study was performed to identify factors influencing primary hemostasis as measured using the Platelet Function Analyzer (PFA-100) system (Siemens Health Care Diagnostics, Marburg, Germany).96 The PFA-100 is an automated instrument developed to assess primary hemostasis under high shear in vitro. In brief, whole blood is aspirated through apertures coated with collagen and adenosine diphosphate (Col-ADP) or collagen and epinephrine (Col-Epi).97 Under shear conditions, these agonists trigger platelet plug formation and thus aperture occlusion. PFA-100 closure times are known to be affected by variables, including platelet number, platelet function, VWF:Ag levels, and VWF activity.97 The GAIT-2 results showed that the ABO locus on chromosome 9 represented the main determinant of PFA-100 closure times for both the Col-Epi and Col-ADP cartridges. In the Col-Epi experiments, all of the ABO effects were attributable to changes in plasma VWF-FVIII levels. In contrast, in the Col-ADP experiments, the influence of ABO was only partially explained by VWF-FVIII levels, suggesting that additional ABO-dependent mechanisms may also be important.96
Second, recent data suggest that ABO expression on GPIb may influence the ability of this glycoprotein to interact with VWF. Dunne et al perfused whole blood over immobilized VWF under arterial shear conditions and then assessed translocation dynamics for blood group O compared with non-O platelets, using video microscopy.98 Interestingly, they observed a significant reduction in interaction of type O platelets with VWF under these conditions. Overall, type O platelets were shown to travel farther over the VWF surface and at significantly greater translocation velocities before they formed stable bonds with VWF. In addition, the fraction of group O platelets that adhered stably to the VWF surface was significantly reduced compared with that of non-O platelets. Subsequent analytical modeling suggested that the differences observed between the different ABO platelet types were very likely related to alterations in GPIb-VWF binding kinetics.98 However, the mechanism through which ABO influences platelet GPIb biology has not yet been defined.99 Additional studies are necessary to investigate whether ABO(H) expression on other platelet membrane receptors influence their functional activities (eg, ability to interact with collagen or fibrinogen). Nevertheless, an ABO effect on platelet function may contribute to the enhanced bleeding observed in patients in group O with low VWF levels.100
Notwithstanding these intriguing questions, it is interesting that 2 previous studies have demonstrated that a subset of healthy individuals in groups A and B show significantly increased levels of A and B antigens expressed on platelet surfaces.94,101 These ABO high-expressing individuals have been estimated to account for up to 7% of the healthy group A or B population. Whether this overexpression of A and B determinants on platelet surface glycoproteins has any physiological or pathological significance remains unclear.
Conclusions
In summary, the association between ABO blood group and risk of thrombosis has been recognized for many years. However, the biological mechanisms through which ABO affects physiological hemostasis and pathological thrombosis have remained relatively poorly understood until recent years. With recent advances, particularly in MS glycomic analysis, exciting developments have been achieved in this clinically important field. In particular, it is clear that ABO blood group has significant quantitative and qualitative effects on many aspects of VWF biology. Furthermore, emerging data suggest that ABO may have previously unrecognized effects in regulating the function of specific platelet membrane receptors. Given the severe morbidity and the mortality that continue to be associated with thrombotic disorders, improved understanding of these glycan-mediated effects may offer the opportunity to develop novel targeted therapeutic strategies.
Acknowledgments
This work was supported by funds from the National Institutes of Health, National Heart, Lung and Blood Institute for the Zimmerman Program (HL081588); a Science Foundation Ireland Principal Investigator Award (11/PI/1066); a Health Research Board Investigator Lead Project Award (ILP-POR-2017-008); and a National Children’s Research Centre Project Award (C/18/1).
Authorship
Contribution: All authors drafted the first version of different sections of the manuscript and all critically reviewed the final manuscript.
Conflict-of-interest disclosure: J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma, Takeda, and Octapharma; has served on the advisory boards of Baxter, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda, and Pfizer; and has received research grant funding awards from Baxter, Bayer, Pfizer, Shire, Takeda, and Novo Nordisk. J.M.O. has received research grant funding from LEO Pharma. S.E.W. declares no competing financial interests.
Correspondence: James S. O’Donnell, Irish Centre for Vascular Biology, Royal College of Surgeons in Ireland, Ardilaun House, 111 St Stephen’s Green, Dublin 2, Ireland; e-mail jamesodonnell@rcsi.ie.
REFERENCES
Author notes
S.E.W. and J.M.O. contributed equally to this review.