Introduction: Daratumumab (DARA) is a CD38-targeting monoclonal antibody approved for the treatment of MM. The addition of DARA to standard-of-care (SOC) regimens in the phase 3 ALCYONE (D-VMP vs VMP) and MAIA (D-Rd vs Rd) studies demonstrated deep and durable responses and improved progression-free survival in transplant-ineligible NDMM patients (pts). DARA-based regimens have shown increased infection rates vs SOC in both ALCYONE (grade 3/4 infections, 22% vs 15%) and MAIA (36% vs 27%). Most common (>10 pts) grade 3/4 infection(s) was pneumonia (D-VMP/VMP; 13%/4%) in ALCYONE and were pneumonia (D-Rd/Rd; 15%/9%), influenza (3%/2%), bronchitis (3%/1%), and sepsis, urinary tract and lower respiratory tract infections (3% each) in MAIA. Most common (>10 pts) serious adverse infection(s) was pneumonia (D-VMP/VMP; 12%/3%) in ALCYONE and pneumonia (D-Rd/Rd; 14%/9%), influenza (4%/2%), bronchitis (4%/2%), and upper respiratory tract infection (3%/3%) in MAIA. Using pooled data from DARA-treated pts in ALCYONE and MAIA, we report results of an analysis to identify predictive markers of grade ≥3 or serious infections that occurred during the first 6 mo of treatment.
Methods: ALCYONE pts received ≥nine 6-week cycles of VMP (V: 1.3 mg/m2 SC on Days 1, 4, 8, 11, 22, 25, 29, and 32 of Cycle 1 and Days 1, 8, 22, and 29 of Cycles 2-9; M: 9 mg/m2 orally, and P: 60 mg/m2 orally on Days 1-4 of Cycles 1-9)±DARA (16 mg/kg IV), QW during Cycle 1, Q3W Cycles 2-9, and Q4W thereafter as maintenance therapy until disease progression or unacceptable toxicity. MAIA pts received 28-day cycles of Rd (R: 25 mg PO once daily on Days 1-21; d: 40 mg PO on Days 1, 8, 15 and 22)±DARA (16 mg/kg IV) QW Cycles 1-2, Q2W Cycles 3-6, and Q4W weeks thereafter. This analysis pooled data for DARA-treated pts in ALCYONE (median follow-up, 40.1 mo) and MAIA (median follow-up, 36.4 mo). Pooled data were randomly split into training and validation data. A predictive model was developed for time to first occurrence of treatment-emergent grade ≥3 or serious infections during the first 6 mo of study treatment.
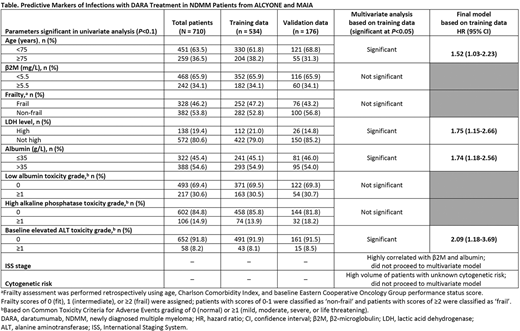
To identify the set of predictors for the Cox proportional hazard multivariate model, each candidate predictor was first assessed in a univariate model. Parameters with a P value <0.1 were selected to fit a multivariate model. Continuous predictors were categorized with clinical input before inclusion in the multivariate model. The final model was developed by dropping parameters that were not significant at P = 0.05, with low Akaike's and Bayesian Information Criteria; multicollinearity was also examined. Once the predictive model was finalized, a scoring tool was developed. Each pt's risk of infection was evaluated through a calculated risk score. The final model and scoring tool were tested using the validation data.
Results: In ALCYONE, a total of 706 pts were randomized (D-VMP, 350; VMP, 356); in MAIA, a total of 737 pts were randomized (D-Rd, 368; Rd, 369). A total of 710 DARA-treated pts (D-VMP, 346; D-Rd, 364) were pooled from both studies to develop the predictive model (training data, 534 pts; validation data, 176 pts). 10 significant parameters were identified from the univariate analysis (Table): age, β2-microglobulin (β2M) level, frailty status, lactic acid dehydrogenase (LDH) level, albumin level at baseline, low albumin level, high alkaline phosphatase level, and elevated baseline alanine aminotransferase (ALT), ISS stage, and cytogenetic risk. The final predictive model included age, LDH, albumin, and elevated baseline ALT levels (Table). Based on the coefficient estimate of each predictor from the final Cox model, a scoring tool was developed with the total score ranging from 0-23; (≥8, high risk; ≤7, low risk). The scoring system was applied to the training data (n = 534); 370 pts were classified at low risk and 164 pts were at high risk for infection. The infection event rate was 15.7% for low-risk pts and 29.3% for high-risk pts (HR, 2.11; 95% CI, 1.44-3.10; P = 0.0001). In the validation data (n = 176), 130 pts were classified as low-risk and 46 pts were at high risk for infection. The infection rate was 16.2% for low-risk vs 28.3% for high-risk pts (HR, 1.91; 95% CI, 0.96-3.83; P = 0.0663).
Conclusion: This analysis identified predictors of grade ≥3 or serious infections during the first 6 mo of treatment in DARA-treated NDMM pts that were used to develop a risk scoring system. These data will be useful in identifying pts most at risk of infection during DARA treatment.
van de Donk:Amgen: Other: Ad Board, Research Funding; BMS: Other: Ad Board, Research Funding; Janssen: Other: Ad Board, Research Funding; Novartis: Other: Ad Board; Bayer: Other: Ad Board; Takeda: Other: Ad Board; Genentech: Other: Ad Board; Celgene: Other: Ad Board, Research Funding. Zweegman:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees. San-Miguel:Amgen, BMS, Celgene, Janssen, MSD, Novartis, Takeda, Sanofi, Roche, Abbvie, GlaxoSmithKline and Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees. Dimopoulos:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau. Cavo:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Honoraria; GlaxoSmithKline: Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Suzuki:Takeda, Amgen, Janssen and Celgene: Consultancy; Takeda, Celgene, ONO, Amgen, Novartis, Sanofi, Bristol-Myers Squibb, AbbVie and Janssen: Honoraria; Bristol-Myers Squibb, Celgene and Amgen: Research Funding. Touzeau:Sanofi: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Takeda: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; GlaxoSmithKline: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Amgen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Usmani:Sanofi: Consultancy, Honoraria, Research Funding; Celgene: Other; Amgen: Consultancy, Honoraria, Other: Speaking Fees, Research Funding; Seattle Genetics: Consultancy, Research Funding; SkylineDX: Consultancy, Research Funding; Incyte: Research Funding; GSK: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Other: Speaking Fees, Research Funding; Takeda: Consultancy, Honoraria, Other: Speaking Fees, Research Funding; Merck: Consultancy, Research Funding; BMS, Celgene: Consultancy, Honoraria, Other: Speaking Fees, Research Funding; Pharmacyclics: Research Funding; Array Biopharma: Research Funding; Abbvie: Consultancy. Perrot:Amgen, BMS/Celgene, Janssen, Sanofi, Takeda: Consultancy, Honoraria, Research Funding. Pei:Janssen: Current Employment, Current equity holder in publicly-traded company. Kudva:Memorial Sloan Kettering Cancer Center: Other: non-paid consultancy; Janssen: Current Employment, Current equity holder in publicly-traded company. Van Rampelbergh:Janssen: Current Employment. Ukropec:Janssen: Current Employment, Current equity holder in publicly-traded company. Leary:Janssen: Current Employment, Current equity holder in publicly-traded company. Carson:Janssen: Current Employment. Qi:Janssen: Current Employment, Current equity holder in publicly-traded company; Johnson and Johnson: Current equity holder in publicly-traded company. Chari:Janssen, Celgene, Novartis, Amgen, Bristol-Myers Squibb, Karyopharm, Sanofi, Genzyme, Seattle Genetics, Oncopeptides, Millennium/Takeda, Antengene, Glaxo Smith Kline, Secura Bio: Consultancy; Janssen, Celgene, Novartis, Amgen, Pharmacyclics, Seattle Genetics, Millennium/Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.