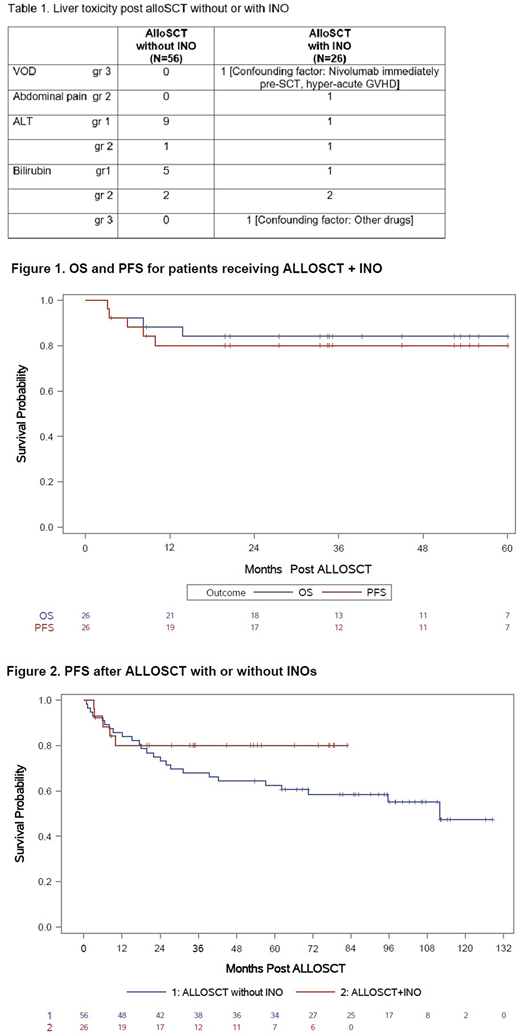
Background: Inotuzumab Ozogamicin (INO) is a humanized anti-CD22 monoclonal antibody that has emerged as an attractive therapeutic target for CD22+ b-cell lymphoid malignancies. We prospectively studied the safety and efficacy of INO when added to our standard nonmyeloablative allogeneic stem cell transplant (alloSCT) conditioning regimen of BFR (bendamustine, fludarabine and rituximab) (Khouri IF, et al. Blood 2014;124:2306). We also compared results to patients who received BFR+alloSCT without INO in prior trial. Methods: INO was infused intravenously (IV) on day -13 outpatient, with a dose cohort of 0.6, 1.2 or 1.8 mg/m2, to determine the maximum tolerated dose. Bendamustine 130 mg/m2 IV daily on days -5 to -3 together with 30 mg/m2 IV of fludarabine on days -5 to -3 were given prior to transplantation. Rituximab was given at a dose of 375 mg/m2 IV on days -6, +1, and +8. Tacrolimus and mini-methotrexate were used for graft versus host disease (GVHD) prophylaxis. In addition, thymoglobulin 1 mg/kg IV was given on days -2, and -1 in patients receiving a matched unrelated donor (MUD) transplant. Results: AlloSCT with INO.The study group included 26 patients treated between December 2012 and September 2019. Median age was 59 (range, 26-70) years. Eleven (42% had an HCT-CI >3. Disease types: CLL [n=7 (27%) ; 2 with TP53 mutations, 2 others with 17p-; 4 with unmutated immunoglobulin heavy chain gene], Richter's (n=4, 15%), mantle cell lymphoma (MCL) [n=8 (31%); 6/6 patients tested had Ki 67 >30%; 1 had blastoid histology and 1 had 17p-], follicular lymphoma (n=5, 19%), and diffuse large b cell (DLBCL[n= 2 (8%); including 1 double-expressor subtype]. Median prior treatments was 2.5 (range, 1-6). CLL patients were previously treated with ibrutinib (n=6, 86%; 5 had the drug discontinued due to refractoriness to ibrutinib, one had poor tolerance, and 1 was bridged to transplant), idelalisib (n=2, refractory), venetoclax (n=4; sensitive), CAR-T cell (n=1, refractory). One patient with Richter refractory to nivolumab+chemotherapy. Four MCL patients were previously treated with ibrutinib (2 had progression, 2 were bridged to transplant). At study entry, 18 (69%) patients were in CR, 7 (27%) in PR, and 1 (4%) had SD. Eleven (42%) received their transplants from HLA-compatible siblings and 15 (58%) from MUDs. The number of patients who received the 0.6, 1.2 or 1.8 mg/m2 of INO were 4, 2 and 20 patients, respectively. No DLT was observed. Neutrophil counts recovered to > 0.5 x 109/L a median of 6 days after transplantation (range, 0-12). Eleven patients (42%) never experienced an ANC < 0.5 x 109/L and 20 (77%) never experienced a platelet counts < 20K x 109/L. All patients engrafted donor cells and no secondary graft failure occurred. By day 30, median donor myeloid and T-cells were 92% and 99%, respectively. Both increased to 100% by day 90. The CI acute grade 2-4 GVHD, 3-4 GVHD and 1-year chronic extensive GVHD were 27%, 4 % (none had grade 4) and 31%, respectively. Non-relapse mortality (NRM) at 5-years was 11.7%. With a median follow-up time of 49 (range, 4-83) months, the 5-year OS and PFS rates were 84% and 81%, respectively (Figure 1). Control group: AlloSCT without INO. We compared results to a group of patients (n=56) with relapsed lymphoid malignancies who received alloSCT at our center on a preceding prospective trial between April 2009 and February 2013, using BFR conditioning without INO and the same GVHD prophylaxis. There was no statistically significant difference in patients, disease or transplant characteristics between the 2 groups. We also found no statistically significant differences in engraftment times, % donor cell, 5-year NRM (12.5%), risk of acute 2-4 or 3-4 GVHD. Liver toxicity encountered between the two groups is summarized in Table 1. The 5-year OS and PFS was 75% and 62%, respectively (Figure 2). Conclusions: Our results show that INO at a dose level of 1.8 mg/m2 is well-tolerated when combined with the BFR nonmyeloablative allogeneic conditioning regimen for lymphoid malignancies. No added toxicity or increased myelosuppression were observed compared to patients who received BFR alone. An ongoing trial at our center includes patients with acute lymphoblastic leukemia evaluating INO added during conditioning and post- alloSCT for maintenance.
Khouri:Bristol Myers Squibb: Research Funding; Pfizer: Research Funding. Jain:Fate Therapeutics: Research Funding; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Aprea Therapeutics: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Precision Bioscienes: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kadia:Abbvie: Honoraria, Research Funding; Cellenkos: Research Funding; Pulmotec: Research Funding; Amgen: Research Funding; Cyclacel: Research Funding; Incyte: Research Funding; BMS: Honoraria, Research Funding; Celgene: Research Funding; Astellas: Research Funding; Ascentage: Research Funding; Pfizer: Honoraria, Research Funding; Astra Zeneca: Research Funding; Novartis: Honoraria; JAZZ: Honoraria, Research Funding; Genentech: Honoraria, Research Funding. Pemmaraju:Roche Diagnostics: Honoraria; Samus Therapeutics: Research Funding; Blueprint Medicines: Honoraria; Celgene: Honoraria; SagerStrong Foundation: Other: Grant Support; LFB Biotechnologies: Honoraria; Novartis: Honoraria, Research Funding; DAVA Oncology: Honoraria; Affymetrix: Other: Grant Support, Research Funding; MustangBio: Honoraria; Daiichi Sankyo: Research Funding; Plexxikon: Research Funding; Incyte Corporation: Honoraria; Cellectis: Research Funding; Stemline Therapeutics: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Pacylex Pharmaceuticals: Consultancy. Bashir:Celgene: Research Funding; StemLine: Research Funding; Acrotech: Research Funding; Takeda: Other: Advisory Board, Research Funding; Purdue: Other: Advisory Board; Amgen: Other: Advisory Board; KITE: Other: Advisory Board. Kebriaei:Novartis: Other: Served on advisory board; Kite: Other: Served on advisory board; Ziopharm: Other: Research Support; Pfizer: Other: Served on advisory board; Jazz: Consultancy; Amgen: Other: Research Support. Popat:Bayer: Research Funding; Novartis: Research Funding. Nastoupil:Genentech, Inc.: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Karus Therapeutics: Research Funding; Gamida Cell: Honoraria; Bayer: Honoraria; Gilead/KITE: Honoraria; Novartis: Honoraria, Research Funding; Merck: Research Funding; Janssen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; TG Therapeutics: Honoraria, Research Funding; LAM Therapeutics: Research Funding. Flowers:BeiGene: Consultancy; Burroughs Wellcome Fund: Research Funding; TG Therapeutics: Research Funding; Bayer: Consultancy; Denovo Biopharma: Consultancy; AbbVie: Consultancy, Research Funding; Acerta: Research Funding; Leukemia and Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; V Foundation: Research Funding; Kite: Research Funding; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; OptumRx: Consultancy; Eastern Cooperative Oncology Group: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; National Cancer Institute: Research Funding; Spectrum: Consultancy; Pharmacyclics/Janssen: Consultancy; Karyopharm: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding. Kantarjian:Novartis: Research Funding; Jazz Pharma: Research Funding; Agios: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Cyclacel: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding; Astex: Research Funding; Takeda: Honoraria; AbbVie: Honoraria, Research Funding; BMS: Research Funding; Ariad: Research Funding. Champlin:Actinium: Consultancy; Takeda: Patents & Royalties; Genzyme: Speakers Bureau; DKMS America: Membership on an entity's Board of Directors or advisory committees; Johnson and Johnson: Consultancy; Omeros: Consultancy; Cytonus: Consultancy. Jabbour:Pfizer: Other: Advisory role, Research Funding; BMS: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; Takeda: Other: Advisory role, Research Funding; Adaptive Biotechnologies: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding; Amgen: Other: Advisory role, Research Funding.
Use Of Inotuzumab Ozogamicin in conditioning for allogeneic transplantation
Author notes
Asterisk with author names denotes non-ASH members.