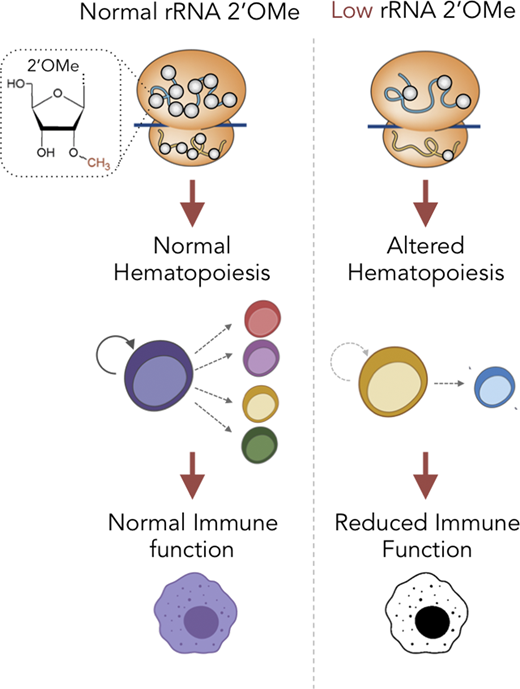
RNA modifications are emerging as key determinants of development and disease. Understanding the mechanisms regulating their functional impact is crucial to uncovering their relevance for disease pathology. The NPM1 gene is frequently a target of genetic alteration in hematological tumors, particularly of the myeloid lineage. While extensively studied, the mechanisms by which NPM1 exert its impact on HSPCs are still not fully elucidated. Recently, we have identified NPM1 as an essential regulator of rRNA 2'-O-methylation (2'OMe). We found that NPM1, through its RNA-binding activity, binds C/D box small nucleolar RNAs (snoRNAs) in the nucleolus, to regulate rRNA 2'OMe, and thereby controls translation. Additionally, we identified NPM1 germ-line mutations in dyskeratosis congenita patients presenting with BMF. Characterization of these mutations found them as selectively deficient in snoRNA binding, and thus their expression led to reduce 2'OMe levels and aberrant translation in patient cells. To causally link reduced 2'OMe to dyskeratosis congenita manifestation, we generated mice harboring a dyskeratosis congenita germ-line NPM1 mutation. The KI mice phenocopy both hematological and non-hematological DC features, thus casually linking mutated NPM1 and reduced 2'OMe to bone marrow failure. Currently, we are taking advantage of our Npm1-mutated mouse model to explore the role of 2'OMe in mature immune cell function. We find that aberrant 2'OMe in mature macrophages (due to Npm1 mutation) does not affect their maturation, however, it leads to altered functions. Specifically, we find that Npm1-mutated macrophages, with reduced 2'OMe, display reduced production of reactive oxygen species, chemotactic properties and phagocytic capacity. These studies demonstrate a role for Npm1 and 2'OMe in adult immune cells, and reveal the importance of translation regulation in both hematopoietic stem cells and mature macrophage function.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.