Background: Outcome in the blast phase of myeloproliferative neoplasm (MPN-BP) is extremely poor with a median overall survival (OS) of 3-6 months. Age > 65 years, transfusion need, platelet count < 100 × 109/L and high-risk karyotype adversely impact the prognosis in MPN-BP (Tefferi, Leukemia, 2018). Diploid karyotype has been rarely reported in patients with MPN-BP, and the outcome remains undefined. We sought to analyze clinical characteristics and outcomes of patients with MPN-BP and diploid karyotype (DP-k).
Methods: We retrospectively evaluated patients (pts) with MPN-BP who presented to our center between 2000-2019. According to cytogenetics, pts were stratified into DP-k and all others (nonDP-k). Only patients with ≥10 metaphases were included. Molecular analysis was performed on some patients using 81-gene panel next - generation sequencing analysis (NGS) (Luthra, Haematologica, 2014). Categorical variables were compared by x2 test. Cox proportional hazards regression was used for associations between variables and OS. The Kaplan-Meier method was used to estimate OS, and it was measured from the date of MPN-BP, till the last follow-up or death. Separate OS analysis was done to censor for stem cell transplantation (SCT). Responses were assessed according to the ELN 2017 AML response criteria (overall response rate, ORR, combination of complete remission with and without counts recovery; CR/CRi).
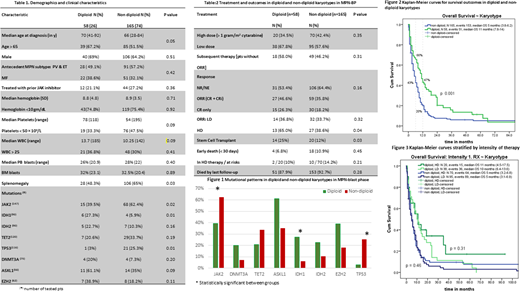
Results: A total of 223 pts with MPN-BP were identified, among them 58 pts (26%) had DP-k. The median age of entire cohort was 67 years and it was similar between groups (p = 0.55). Demographic and clinical data are summarized in Table 1. Among clinical features, we only observed differences in the frequency of palpable splenomegaly; less common in pts with DP-k (48% vs 65 %, p = 0.03). Patients with DP-k harbored less frequently: JAK2 (38% vs 62%, p = 0.02) or TP53 mutations (3% vs 25%, p=0.01), but enriched with epigenetic mutations (e.g., IDH1, ASXL1, EZH2; Table 1).
Table 2 shows treatment details and outcomes. All patients received at least one course of induction therapy. Intensive chemotherapy (≥1 gram of cytarabine per m2; high dose, HD) was used less frequently than other approaches (low dose, LD), but it was not statistically significant (40% vs 60%, respectively; P=0.48). Intensity of treatment regimens also did not vary among groups (HD in DP-k vs nonDP-k, 35% vs 42%, respectively, p = 0.35). The ORR was 38.5% and it was comparable between groups (47% vs 36% for DP-k vs nonDP-k, p = 0.16). Patients with DP-k had higher ORR with HD therapy (65% vs 39%, respectively, p = 0.04).
Among non-responders to induction (n=137), 67 patients (48.9%) received subsequent salvage therapy (similar for DPk vs non-DP-k, 58% vs 46%, p 0.30). However, more pts with DP-k were able to receive more than 1 salvage therapy or underwent SCT (Table 2).
Early mortality (by day 30) was 9.8% for the entire cohort and was similar between groups (7% vs 11%, p = 0.34) regardless of the intensity of regimen (Table 2). Over the median follow-up of 6.3 months (range, 2-154), 204 (91.5%) patients died (88% vs 93% for DP-k vs nonDP-k, p = 0.28).
The median OS for all pts was 6 months (95% CI 4.5-7.4 months). Pts with DP-k had superior OS than nonDP-k with respective medians of 11 [95% CI 7.8-14.1] and 5 months [95% CI 3.8-6.2], p = 0.001, HR = 0.61, 95% CI: 0.44-0.84), Figure 2. Pts with DP-k had higher survival rates at 6 and 12 months from MPN-BP than those with nonDP-k (Figure 2). OS censored for SCT showed similar results with medians of 11 and 4 months, respectively, p<0.001, HR 0.53, 95% CI 0.37-0.76. Intensity of therapy did not affect OS in either group (Figure 3).
In univariable analysis, platelets < 50 × 109/L, mutations in DNMT3A, TET2, IDH1, TP53, and lack of response to induction therapy predicted for inferior outcome in patients with nonDP-k. We did not find any significant predictors for those with DP-k, but pts with no additional mutation, presence of single ASXL1 or IDH1/2 mutation had favorable OS with medians of 14, 12, and 11 months, respectively.
Conclusions: Pts with DP-k MPN-BP appear to have distinct disease biology from those with other karyotypic abnormalities. Despite median age of 70 years, 47% of these pts were alive at 12 months from MPN-BP diagnosis. Careful therapeutic decisions are required to mitigate treatment-related toxicity and improve prognosis in this rare subgroup of patients with DP-k and MPN-BP.
Verstovsek:Sierra Oncology: Consultancy, Research Funding; AstraZeneca: Research Funding; Gilead: Research Funding; Celgene: Consultancy, Research Funding; NS Pharma: Research Funding; CTI Biopharma Corp: Research Funding; Genentech: Research Funding; Blueprint Medicines Corp: Research Funding; Novartis: Consultancy, Research Funding; Incyte Corporation: Consultancy, Research Funding; Protagonist Therapeutics: Research Funding; PharmaEssentia: Research Funding; Promedior: Research Funding; Roche: Research Funding; ItalPharma: Research Funding. Bose:Astellas Pharmaceuticals: Research Funding; Incyte Corporation: Consultancy, Honoraria, Research Funding, Speakers Bureau; Promedior, Inc.: Research Funding; Constellation Pharmaceuticals: Research Funding; CTI BioPharma: Honoraria, Research Funding; Kartos Therapeutics: Honoraria, Research Funding; Celgene Corporation: Honoraria, Research Funding; Pfizer, Inc.: Research Funding; NS Pharma: Research Funding; Blueprint Medicines Corporation: Honoraria, Research Funding. Pemmaraju:Affymetrix: Other: Grant Support, Research Funding; AbbVie: Honoraria, Research Funding; Daiichi Sankyo: Research Funding; Novartis: Honoraria, Research Funding; Incyte Corporation: Honoraria; DAVA Oncology: Honoraria; Samus Therapeutics: Research Funding; Plexxikon: Research Funding; Stemline Therapeutics: Honoraria, Research Funding; Celgene: Honoraria; Cellectis: Research Funding; MustangBio: Honoraria; Blueprint Medicines: Honoraria; LFB Biotechnologies: Honoraria; Pacylex Pharmaceuticals: Consultancy; Roche Diagnostics: Honoraria; SagerStrong Foundation: Other: Grant Support. Kantarjian:Immunogen: Research Funding; Agios: Honoraria, Research Funding; Ariad: Research Funding; Amgen: Honoraria, Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Research Funding; BMS: Research Funding; Cyclacel: Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Research Funding; Takeda: Honoraria; Astex: Research Funding; Daiichi-Sankyo: Research Funding; AbbVie: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.