Introduction:
Our group and others have previously shown that the presence of complex karyotype (>/= 3 cytogenetic abnormalities) is an important prognostic factor in relapsed/refractory (RR) chronic lymphocytic leukemia (CLL) patients treated with ibrutinib (Woyach JCO 2017, Thompson Cancer 2015, Maddocks JAMA Oncology 2015). It has been shown recently that increasing karyotypic complexity is a prognostic marker (Baliakis Blood 2019), but whether this is relevant for patients treated with novel therapies is unclear. Here, we aimed to determine whether the degree of karyotypic complexity beyond the dichotomy of complex versus not is a prognostic variable for patients with CLL treated with ibrutinib.
Methods:
We conducted a retrospective analysis of all patients with CLL treated with ibrutinib as a single agent or in combination with a monoclonal anti-CD20 antibody (MOAB) from 2010 through 2019 at The Ohio State University. We included patients with both treatment-naïve (TN) and RR disease. To determine karyotype, cells were stimulated with mitogen cocktail (PWM/PMA/CpG-ODN) and analyzed according to standard laboratory procedures. FISH using probes for D13S319, D12Z3, ATM, and TP53 were done according to manufacturer's recommendations. PCR was used to determine IGHV mutational status. Cytogenetic testing was included in the analysis if done <6 months before or within 60 days after ibrutinib initiation. All characteristics were measured at ibrutinib start. Multivariable Cox proportional hazards models were built to correlate karyotypic complexity (defined as a continuous variable with 1 unit increases) with progression free survival (PFS) and overall survival (OS), adjusted for age, RR status, ECOG performance status (PS), lactate dehydrogenase (LDH), white blood cell (WBC) count, hemoglobin (HgB), platelet (PLT) count, presence of del17p13 and IGHV mutational status. Multivariable models were fit in imputed datasets, and estimates were obtained from combining results from across 30 imputed datasets.
Results:
We analyzed 561 patients with a median age of 65 (range 26-91). 86% were treated with ibrutinib monotherapy, 22% were TN, and median number of prior therapies was 2 (range 0-13). 96% had an ECOG performance status (PS) of 0 or 1. Median LDH, WBC, HgB and PLT were 213 U/L, 23.2 K/uL, 11.2 g/dL, and 115 K/uL respectively. With available data, del13q14, trisomy 12, del11q22, and del17p13 was present in 50%, 22%, 30%, and 29% of patients respectively; 74% of patients were IGHV unmutated. 63 patients were excluded from cytogenetic analyses as the test was not done during the specified time window. Of the 458 evaluable, 50% had >3 cytogenetic abnormalities including 30% with >5 abnormalities. After a median follow up of 55.5 months, for the entire cohort estimated median PFS was 61.6 months (95% CI 56.1-69.5), and estimated median OS was 95.4 months (95% CI 86.3-not reached).
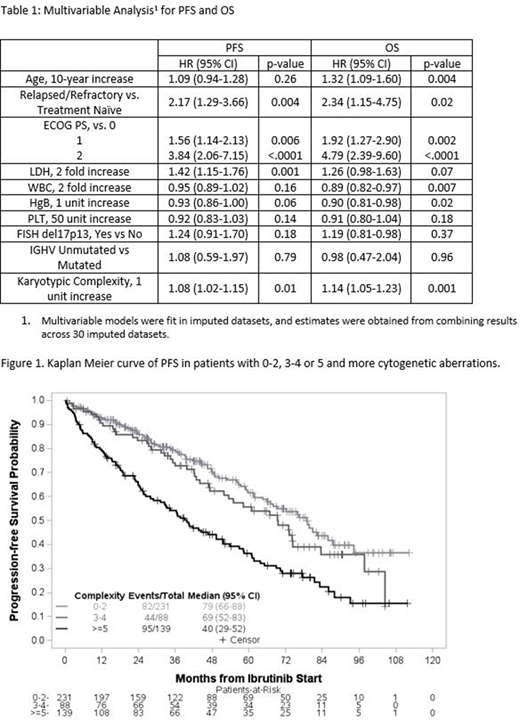
On univariable analysis, older age, RR status, higher ECOG PS and LDH, lower HgB and PLT, presence of del17p13 and increasing karyotypic complexity were found to be statistically significant predictors of worse PFS and OS. To illustrate the relationship between increasing karyotypic complexity and clinical outcome, Kaplan-Meier plots are provided grouping pts with 0-2, 3-4, and 5 or higher aberrations (Figure 1). Accounting for statistically and clinically important factors on multivariate analysis (MVA, Table 1), increasing karyotypic complexity continued to be a statistically significant predictor of both PFS (p=0.01, HR 1.08 (95% CI 1.02-1.15)) and OS (p=0.001, HR 1.14 (95% CI 1.05-1.23)). Del17p13 did not retain statistical significance independently of karyotypic complexity on MVA. The interaction effect between prior treatment status and karyotypic complexity term was not significant for OS (p=0.89) and PFS (p=0.46), suggesting the prognostic effect of karyotypic complexity is similar across TN and RR cohorts.
Conclusions:
In this single center retrospective analysis, we found that increasing karyotypic complexity predicts inferior survival for patients with CLL treated with ibrutinib. This highlights that it is not only important to dichotomize presence of complex karyotype as >3 abnormalities, but to describe the number of karyotypic abnormalities in subsequent studies.
Bond:Seattle Genetics: Honoraria. Byrd:Acerta Pharma: Research Funding; Trillium: Research Funding; Pharmacyclics LLC, an AbbVie Company, Janssen, Novartis, Gilead, TG Therapeutics: Other; Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, Novartis, Janssen: Speakers Bureau; Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, BeiGene: Research Funding; Janssen: Consultancy; Leukemia and Lymphoma Society: Other; Novartis: Research Funding; Kartos Therapeutics: Research Funding; Vincera: Research Funding; Syndax: Research Funding. Rogers:AstraZeneca: Other: Travel; Abbvie, Acerta, AstraZeneca, Pharmacyclics: Consultancy; Abbvie, Genetech, Janssen: Research Funding. Woyach:Pharmacyclics: Consultancy, Research Funding; AbbVie: Research Funding; Janssen: Consultancy, Research Funding; Karyopharm: Research Funding; Morphosys: Research Funding; Loxo: Research Funding; Verastem: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.