CD19 is an attractive therapeutic target as it is highly expressed in CLL. Tafasitamab (Taf) is an Fc-engineered, humanized anti-CD19 monoclonal antibody with efficacy in CLL as a single agent. Compared to non-engineered antibodies, Taf shows significantly increased antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis, and direct cytotoxic effects (apoptosis) on tumor cells. With the addition of lenalidomide (Len), ADCC can be further augmented in vitro. Given the potential synergy of these agents, acceptable individual safety profiles, and efficacy as single agents, we conducted a study of Taf in combination with Len in patients (pts) with treatment-naïve (TN) and relapsed/refractory (RR) CLL, and included a pilot cohort of ibrutinib (IB)-treated pts with resistance mutations, but no clinical relapse, with Taf added to IB.
We present the final results of this single institution phase II trial. In combination with Len, Taf was given at a dose of 1 mg/kg on cycle(C) 1 day(D) 1, 9 mg/kg on C1 D 2, 8, 15, and 22, and D1 of C2-12. Len 2.5 mg began daily on C1D8 and was given continuously. Len was escalated to 10 mg daily in pts without toxicity. After C12, Len could continue indefinitely in responding pts. In combination with IB, Taf was given at 1 mg/kg on C1D1, 12 mg/kg on C1 D2, 8, 15, and 22, weekly during C2-3, and every other week through C12. IB continued at 420 mg daily. The primary endpoint for Phase II was overall response rate (ORR) after C6. Based on previous studies with single agent Len, we tested whether the combination could improve ORR, from 50% to 80% in the TN cohort, and from 30% to 60% in the RR cohort. Using single stage designs, 19 pts per cohort provided at least 90% power to detect an increase in ORR (type I error rate 10%). By design, 13 and 9 responses were needed in the TN and RR cohorts, respectively, to warrant further study. A secondary endpoint was ORR after 12 cycles of therapy.
Twelve pts were enrolled in the TN cohort; median age was 62 (range 44-75). Six pts were Rai stage III/IV, and 1 had both del(17p) and del(11q). Nine pts had ZAP 70-methylated disease. After C6, ORR was 67% [1 complete response (CR), 7 partial responses (PR); 90% confidence interval (CI): 39-88]; responses remained the same after C12. Three pts completed treatment per protocol, 3 discontinued treatment for progressive disease (PD) and 6 for AEs.
Thirteen pts were enrolled in the RR cohort, median age was 70 (range 62-75). Eight pts were Rai stage III/IV, 4 had del(17p) and 2 had del(11q). Eleven pts had ZAP 70-methylated disease. After C6, ORR was 15% (all PRs - 90% CI: 3-41), and responses improved to 38% after C12 (90% CI: 17-65). Additional 1 pt had stable disease (SD). Five pts completed treatment per protocol, 6 discontinued treatment for PD, 1 for alternative therapy, and 1 continues on treatment.
Nine pts with molecular progression on IB were enrolled; median age was 62 (range 45-87). Seven pts had del(17p) and 1 pt had del(11q). Eight pts had ZAP 70-methylated disease. After C6, only 1 pt (11%) responded with a PR; 3 additional pts had SD. Two pts completed treatment per protocol criteria, 3 discontinued treatment for AEs, 2 for alternative therapy and 1 pt each for PD and increased C481S allelic frequency.
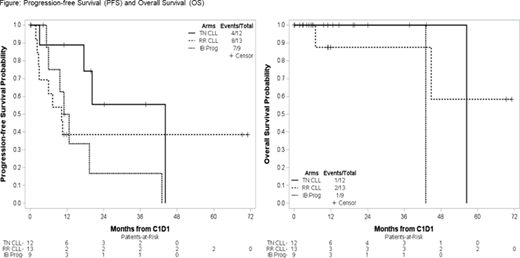
Figure shows Progression-free Survival (PFS) and Overall Survival (OS) for the 3 cohorts.
Taf plus Len was well tolerated. Grade(gr)≥3 AEs included: infections [4: I each of lung infection, sinusitis, upper respiratory tract infection (URTI) and appendicitis], decreased neutrophil count (3) and infusion related reactions (2) in TN pts and decreased neutrophil count (10), infections [7: 2 catheter related urinary tract infections, 1 each with URTI, lung infection and soft tissue infection, 2 unspecified], diarrhea (3), hyponatremia (3) and hypophosphatemia (3) in RR pts. In the Taf plus IB cohort most common gr≥3 AEs included infections (4: 2 lung infections, 1 URTI and I unspecified), hyperglycemia (3) and hyponatremia (3). Gr≥3 atrial fibrillation was seen in 1 pt.
In conclusion, although the trial did not fully accrue as planned, Taf in combination with Len appeared safe and demonstrated promising activity in TN CLL, warranting further investigation, and has similar efficacy to rituximab plus Len in RR CLL. Taf plus IB was less effective in pts with resistance to IB.
Blachly:AbbVie, AstraZeneca, KITE Pharma: Consultancy. Rogers:AbbVie: Consultancy, Research Funding; Genentech: Research Funding; Janssen: Research Funding; Pharmacyclics: Consultancy; Acerta Pharma: Consultancy; AstraZeneca: Consultancy, Other: Travel Funding. Lozanski:Genentech, Novartis, Beckman Coulter: Research Funding. Byrd:Syndax: Research Funding; Vincera: Research Funding; Acerta Pharma: Research Funding; Novartis: Research Funding; Kartos Therapeutics: Research Funding; Trillium: Research Funding; Leukemia and Lymphoma Society: Other; Janssen: Consultancy; Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, BeiGene: Research Funding; Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, Novartis, Janssen: Speakers Bureau; Pharmacyclics LLC, an AbbVie Company, Janssen, Novartis, Gilead, TG Therapeutics: Other. Woyach:Karyopharm: Research Funding; Morphosys: Research Funding; Pharmacyclics: Consultancy, Research Funding; Verastem: Research Funding; AbbVie: Research Funding; Janssen: Consultancy, Research Funding; Loxo: Research Funding.
Lenalidomide off label use for CLL
Author notes
Asterisk with author names denotes non-ASH members.