INTRODUCTION: Pediatric acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) are heterogeneous diseases mediated by changes in protein expression. As most chemotherapeutic agents target proteins, and because overall survival of pediatric AML is far inferior to both pre-B and T-ALL, we aimed to compare the proteomic landscape of pediatric T-ALL and AML, with the goal of determining common AML-T-ALL pathways that are potentially targetable with novel agents.
METHODS: Reverse phase protein arrays (RPPA) analysis was used to measure protein expression in 858 acute leukemia samples (358 T-ALL and 500 AML, 723 pediatric (< 18 yrs.), 135 adults (≥18 yrs.)) and 61 normal CD34+ samples using 270 validated antibodies. Expression levels were normalized against CD34+ cells. Proteins were allocated into 30 functionally related subgroups (Protein Functional Group (PFG)). A progeny clustering algorithm was applied to each PFG to search for strong correlations between proteins and to identify an optimal number of Protein Clusters (PC). Block clustering identified PC that recurrently co-occurred together (Protein Constellation (CON)) and patients that expressed similar combination of CON were defined as Protein Signature (SIG). Proteins that were differentially expressed were identified using the Student's t-test or ANOVA, with a Bonferroni adjusted p-value (0.05/ 270 = 0.00019)).
RESULTS: Of the 270 analyzed proteins, 131 proteins (49%) were differentially expressed between T-ALL and AML; 60 were higher in T-ALL, 71 in AML. Similar to our previous analysis in adult AML and ALL, cell cycle regulators (CDKN1A, CDKN1B) and 2 of the 5 histone marks (H3K36Me3 & H3K4Me3) were higher expressed in T-ALL compared to AML. Heat shock proteins (HSP90AA1_B1, HSPA1A_L, HSPB1 and HSPB1-pSer82) were higher in AML as well as translation proteins EIF2S1, EIF4E and EIF4EBP1 and ribosomal proteins RPS6-pSer235_236 and RPS6KB1, while expression of the translation inhibitory proteins EIF2S1-pSer51 and EIF2AK2-pThr451 was lower in AML compared to T-ALL.
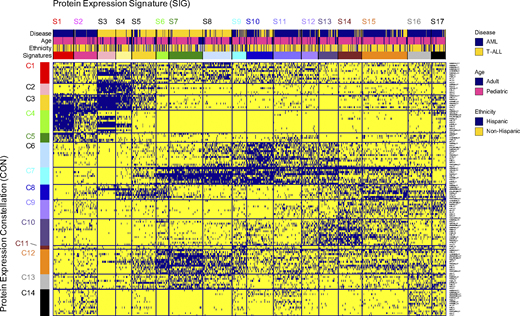
Next, cluster analysis in the context of 30 PFG resulted in 133 PC. The majority (n=102) of PC were expressed in both diseases, 30 PC (22.6%) were AML-specific, and only one PC was specific to T-ALL (characterized by high CDKN1A, CDKN1B and CCND1, but low WEE1, CCNB1 and RB1-pSer). Co-clustering of the 133 PC identified 14 CON that formed 17 SIG. Three CON (5, 9, 10) were associated with AML, 2 with T-ALL (2, 13) and 8 CON were observed in both diseases. In contrast, 15 of SIG were associated with either T-ALL or AML, and two SIG (9, 10) included a mixture of both diseases (P < 0.001, annotation bar Figure 1 "Disease")). SIG were associated with gender (P < 0.001), but not with CNS-status and ethnicity (Hispanic vs. non-Hispanic). No age-specific (kids vs. adults) signatures were observed. For each SIG and CON, proteins significantly higher or lower expressed compared to the normal CD34+ cells were identified.
CONCLUSIONS: This study provides support for our previous hypothesis that pediatric T-ALL and AML can be characterized by recurrent protein expression patterns. While most PC and CON were found in both diseases, SIG (i.e. combinations of protein expression patterns) were specific to either T-ALL or AML. We found similar results when comparing B-ALL to AML in adults. Shared CON indicate that there are common protein expression patterns between pediatric T-ALL and AML. Proteins or pathways with similar utilization (e.g. CON3, 5) in both diseases may allow for information on clinical utility from one disease to be applicable to the other. Those with differential utilization are likely to be uninformative with respect to clinical utility in the other disease.
Figure. "MetaGalaxy" analysis for pediatric AML and T-ALL. Each row represents one protein clusters (n = 133), each column represents one patient (n = 858). Blue indicates membership for that particular protein cluster. Annotation bar shows strong correlation with disease (yellow = T-ALL, blue = AML). No associations were seen for age (blue = adult, pink = pediatric) or Ethnicity (blue = Hispanic, yellow = non-Hispanic).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.