Background: B-cell Acute Lymphoblastic Leukemia (B-ALL) represents an aggressive malignancy but highly curable in children. Currently, pediatric collaborative clinical trials have reported survival rates that exceed 90%, and DNA ploidy by flow cytometry (FC) has been pointed for risk stratification and prognosis in these clinical trials. However, its generalized use remains controversial as few studies reported no impact in real-world populations. We aimed to evaluate prognostic value of DNA ploidy measured by FC in a large cohort of Peruvian children with B-ALL.
Methods: We evaluated prospectively DNA-ploidy by FC in bone marrow diagnostic samples from newly diagnosed children (<15 years) with B-ALL treated at Instituto Nacional de Enfermedades Neoplasicas (Lima-Peru) between 2017-2019. DNA-ploidy was evaluated using Propidium Iodide and calculated as the mean ratio between fluorescence of pathologic B blasts and normal marrow cells. Ploidy categories were established based on previous reports of DNA-index(DI): Diploid + Low-Hyperdiploidy (DLH; DI: 0.95 - 1.15), hypo-diploid (HD; DI<0.95) and High-hyperdiploidy (HH; DI>1.15), and recalculated using maximally selected rank statistics. Samples were analyzed in a FacsCanto II flow cytometer (BD) and Infinicyt software (Cytognos). All patients received BFM-2009 protocol and had minimal residual disease evaluation at the end of IA and IB induction. Minimal residual disease (MRD) was evaluated with FC using a detection threshold of 0.0025% and considering positive ≥0.01%. Survival curves (event-free and overall survival) were estimated using the Kaplan-Meier method and compared with the Log-rank test.
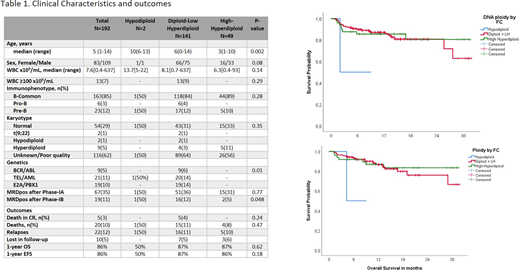
Results: A total of 192 children were included (2 HD, 141 DLH and 49 HH cases according to DNA ploidy by FC). Clinical characteristics and outcomes are shown in Table 1. Median age at diagnosis was 5 years (Range:1-14), 10 years for HD, 6 and 3 years for DLH and HH, respectively (p=0.002). F/M ratio was 1:1.3 for all cases, but 1:2 in HH group. Most karyotypes (62%) had unsatisfactory or poor-quality result, 29% were considered normal, and only 9 hyperdiploidy and 2 hypodiploidy cases were detected by conventional karyotyping. Regarding genetics, 21 TEL/AML, 19 E2A/PBX1 and 9 BCR/ABL cases were detected by multiplex-PCR and most balanced alterations had DLH subtype and only one HD case had TEL/AML. MRD positivity after Induction IA was 35% without difference between groups (50% HD, 36% DLH and 31% HH, p=0.77), however after Induction IB, MRD was positive in 50% of HD, 12% of DLH and 5% of HH (p=0.048). At eight-teen months of follow-up, one relapse was seen in HD cases, and 11% DLH and 10% in HH. Median EFS and OS was not reached, however one-year EFS and OS were 86% for both without significant differences between groups. Multivariate analysis showed that MRD positivity remains as the principal independent prognostic factor and ploidy by DNA did not show any impact in terms of MRD, EFS and OS.
Conclusion: High-Hyperdiploidy by DNA ploidy was associated to better MRD negativity rate after induction IB but without impact in short-term EFS and OS. DNA ploidy did not represent a prognostic factor in our study cohort, however long-term follow-up is warranted. Additionally, a better genetic risk stratification is necessary to improve outcomes in Latino high-risk population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.