Background: There are limited therapeutic options for patients (pts) with myelofibrosis (MF) who lose response to ruxolitinib (Rux). The combination of navitoclax (Nav) plus Rux (NCT03222609) has been shown to induce clinically meaningful spleen volume (SV) responses, improvement in Total Symptom Score (TSS), and reduction in bone marrow fibrosis (BMF) grading in pts with MF who no longer benefit from Rux. At diagnosis, pts with primary MF who have high-molecular-risk (HMR) mutations, defined as mutations in ASXL1, SRSF2, EZH2, U2AF1, and IDH1/2, have shorter overall survival and/or increased risk of leukemic transformation. In addition to mutations in HMR genes, the total number of all genes mutated (regardless of whether they are HMR genes) also correlates with reduced SV responses, treatment (Tx) duration, and overall survival. This phase 2 study explored whether the presence of HMR, or the total number of genes mutated at study entry, correlated with clinical outcomes (SV reduction ≥35% [SVR35], reductions in TSS and BMF) and reductions in driver gene (JAK2 p.V617F and mutated CALR) variant allele frequency (VAF) following Nav plus Rux. As pts with MF have demonstrated a markedly abnormal cytokine profile at baseline, the ability of Nav plus Rux to mediate known MF inflammatory cytokines was also assessed.
Methods: MF pts with Rux failure who had received ≥12 weeks of continuous Rux and had persistent splenomegaly that required a new Tx were enrolled. Pts continued Rux, and Nav was started at 50 mg QD with stepwise escalation to 300 mg on the basis of tolerability. Study endpoints included SVR35 (by MRI), change in TSS (MF Symptom Assessment Form version 4.0) at week (Wk) 24, and change in BMF (locally assessed). At baseline and Wk 24, mutational analyses including VAF measurement were performed in peripheral blood by next-generation sequencing with the 54-gene Focus::Myeloid™ panel (3% limit of detection). At baseline, Wk 12 and 24, levels of inflammatory cytokines were measured in plasma with the 133-analyte ExplorerMAP™ panel.
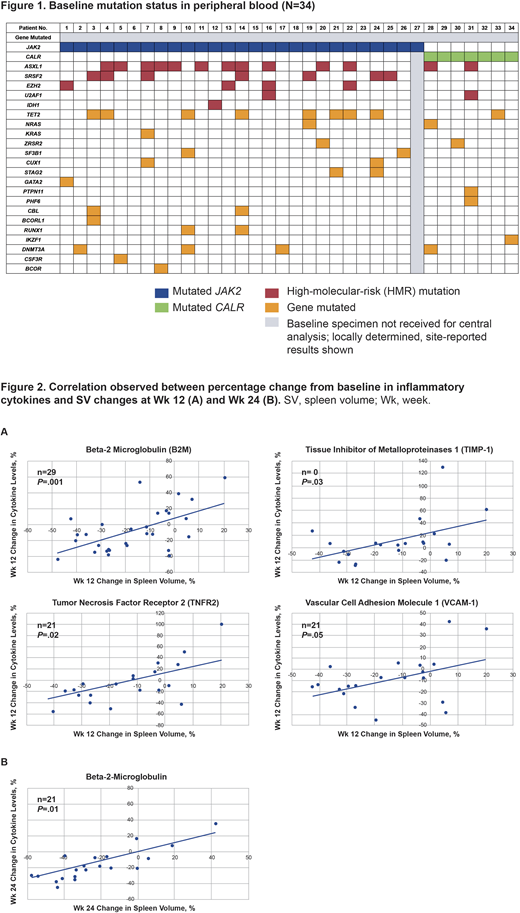
Results: As of February 28, 2020, 34 pts with MF had received ≥1 dose of Nav plus Rux. At study entry, 33 pts were evaluable for biomarker analysis. JAK2 was mutated in 26/33 (79%) pts and 7/33 (21%) pts were positive for mutated CALR. Median VAFs for JAK2- and CALR-mutated pts were 88% and 39%, respectively. Baseline mutational analysis revealed the presence of HMR genes in 19/33 (58%) pts; of these, 8/19 (42%) had ≥2 HMR genes mutated. Mutation rates for ASXL1, SRSF2, EZH2, U2AF1, and IDH1 were 13/19 (68%), 7/19 (37%), 4/19 (21%), 2/19 (10%), and 1/19 (5%), respectively (Figure 1). At baseline, the median number of all genes mutated was 3, and 17/33 (52%) pts harbored ≥3 mutations. At Wk 24, among evaluable pts, 9/34 (27%) achieved SVR35, 6/20 (30%) reached ≥50% reduction in TSS (TSS50), 7/34 (21%) had -1/-2 grade improvement in BMF, and 12/26 (46%) had >10% driver gene VAF reductions. Pts who achieved either SVR35, TSS50, BMF improvement, or VAF reduction were assessed and the distribution of pts with or without HMR mutations, and with ≥3 or <3 total genes mutated was determined. Wk 24 clinical responses and VAF reductions were observed independent of the presence of HMR mutations and number of genes mutated. Notably, 5/9 (56%) pts who achieved SVR35 at Wk 24 had HMR mutations. The frequency of pts with HMR mutations or ≥3 mutations in responders is consistent with that observed in the entire cohort.
Further analyses revealed a direct correlation between changes from baseline in known MF-associated cytokines and SV changes (Figure 2A and 2B) (ie, beta-2 microglobulin [B2M; Wk 12 and 24], tumor necrosis factor receptor 2 [TNFR2; Wk 12], tissue inhibitor of metalloproteinases 1 [TIMP-1; Wk 12], and vascular cell adhesion molecule 1 [VCAM-1; Wk 12]). The Wk 24 TNFR2, TIMP-1, and VCAM-1 analyses will be available for presentation. These 4 cytokines have also been shown historically to correlate with TSS improvement.
Conclusions: Pts with MF previously treated with Rux who then receive Nav plus Rux in combination achieved clinically meaningful SVR, TSS improvement, reduction in BMF, and driver gene VAF reductions independent of HMR mutations and the total number of genes mutated. Ongoing cytokine analyses suggest that the combination of Nav plus Rux may have a role in modulating key cytokines implicated in TSS improvement in pts with MF with suboptimal response to Rux alone.
Pemmaraju:MustangBio: Honoraria; Pacylex Pharmaceuticals: Consultancy; Samus Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; Cellectis: Research Funding; Plexxikon: Research Funding; Affymetrix: Other: Grant Support, Research Funding; Novartis: Honoraria, Research Funding; Roche Diagnostics: Honoraria; Blueprint Medicines: Honoraria; Stemline Therapeutics: Honoraria, Research Funding; Celgene: Honoraria; AbbVie: Honoraria, Research Funding; Incyte Corporation: Honoraria; DAVA Oncology: Honoraria; SagerStrong Foundation: Other: Grant Support; LFB Biotechnologies: Honoraria. Garcia:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Pfizer: Research Funding; Eli Lily: Research Funding. Potluri:AbbVie: Current Employment, Other: may hold stock or stock options. Holes:AbbVie Inc.: Current Employment, Current equity holder in publicly-traded company. Harb:AbbVie: Current Employment, Other: may hold stock or stock options. Jung:AbbVie Inc.: Current Employment, Current equity holder in publicly-traded company. Hutti:AbbVie Inc.: Current Employment, Other: may hold stock or stock options. Verstovsek:Blueprint Medicines Corp: Research Funding; Gilead: Research Funding; Promedior: Research Funding; Genentech: Research Funding; Roche: Research Funding; AstraZeneca: Research Funding; ItalPharma: Research Funding; Incyte Corporation: Consultancy, Research Funding; CTI Biopharma Corp: Research Funding; Protagonist Therapeutics: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; PharmaEssentia: Research Funding. Harrison:CTI Biopharma Corp: Honoraria, Speakers Bureau; Janssen: Speakers Bureau; Gilead Sciences: Honoraria, Speakers Bureau; Incyte Corporation: Speakers Bureau; Sierra Oncology: Honoraria; Shire: Honoraria, Speakers Bureau; AOP Orphan Pharmaceuticals: Honoraria; Roche: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau; Celgene: Honoraria, Research Funding, Speakers Bureau; Promedior: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.