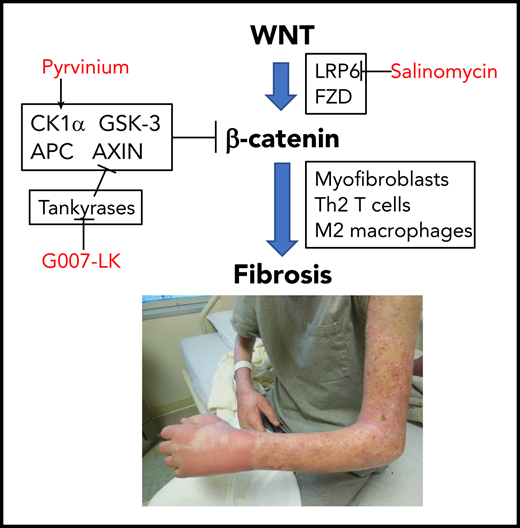
In this issue of Blood, Zhang et al report that the WNT signaling pathway is involved in human and mouse sclerotic chronic graft-versus-host disease (cGVHD) and can be blocked by available inhibitors, providing support for targeting this pathway therapeutically.1
Zhang et al propose that aberrant canonical WNT signaling in cGVHD leads to fibrosis and is blocked by WNT pathway inhibitors.
Zhang et al propose that aberrant canonical WNT signaling in cGVHD leads to fibrosis and is blocked by WNT pathway inhibitors.
Sclerotic cGVHD of the skin, fascia, lung, or other organs is a highly morbid complication of allogeneic hematopoietic cell transplantation (HCT), with poor treatment options. Sclerotic manifestations occur in up to 20% of people with cGVHD and cause thickened skin and subcutaneous fibrosis, joint limitations, obstructive lung changes, and other restrictions.2 Advanced cases cause pulmonary insufficiency resulting from truncal sclerosis or bronchiolitis obliterans syndrome, limited mobility, and nonhealing skin ulcers. Although skin sclerosis is not statistically associated with worse survival,3 bronchiolitis obliterans syndrome has a 50% mortality rate by 5 years after diagnosis.4 A better understanding of the underlying pathophysiology and more effective preventive and treatment options for these forms of cGVHD are desperately needed.
Some readers may not be surprised by this report, because involvement of the WNT signaling pathway has been extensively investigated in other forms of sclerosis, such as systemic sclerosis, and indirect evidence of WNT signaling involvement in cGVHD was previously reported.5 However, Zhang et al provide multiple lines of direct evidence showing canonical WNT signaling is involved in human and murine sclerotic cGVHD and targetable with available agents.
In 2 minor antigen–mismatched murine models, administration of 3 different WNT signaling pathway inhibitors prevented skin fibrosis and decreased infiltration of inflammatory T cells, B cells, and macrophages (see figure). Intriguingly, there was also some evidence of decreased pulmonary fibrosis in these models, suggesting theoretical efficacy against bronchiolitis obliterans, although no functional data are presented. Skin biopsies from people with sclerotic cGVHD showed an increase in nuclear β-catenin and overexpression of WNT-related pathway genes that were not seen in the skin of patients without cGVHD undergoing transplantation.
Of note, Zhang et al only tested WNT pathway inhibitors for prevention; it is not clear if fibrosis is reversible once established or whether continuous treatment is needed, which may be difficult to deliver given potential toxicities of the available agents with long-term administration. Nevertheless, this report provides the preliminary background and justification for designing clinical trials of early treatment of sclerotic cGVHD. Relevant to clinical translation, of the 3 different WNT pathway inhibitors that were tested in the mouse models, pyrvinium is already approved as treatment for pinworms, G007-LK is in cancer clinical trials, and salinomycin is used as an antibiotic in veterinary medicine. Other WNT pathway inhibitors are also in clinical trials for different indications.5 Because the WNT pathway is involved in wound healing and control of the gastrointestinal and hematopoietic stem cell compartment, any human trials will need to monitor closely for adverse events, including relapse of the underlying malignancy.
Finally, therapeutics such as WNT pathway inhibitors that act on target tissues rather than broadly suppressing the immune system are appealing because they may prevent tissue damage resulting from cGVHD while preserving pathogen response and anticancer effects. However, such treatments would need to be continued long term until the systemic alloimmune response is otherwise controlled, because they do not fundamentally promote tolerance. For a manifestation like sclerosis that tends to develop in a minority of patients quite late after HCT, it is not practicable to test a prophylactic approach, because this would require long exposure and overtreatment of many patients. Instead, future clinical trials should focus on very early treatment of sclerosis to see if the most disabling manifestations can be prevented.
Conflict-of-interest disclosure: S.J.L. reports research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, and Takeda and steering committee participation for Incyte.