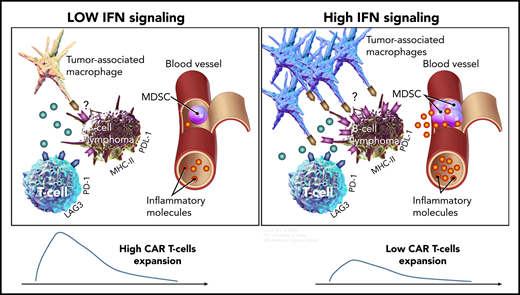
Compared with B-cell lymphomas with low IFN signaling (left), high tumor IFN signaling (right) is associated with a higher number of tumor-associated macrophages, a higher level of systemic inflammatory molecules, and increased expression of immune checkpoint ligands, such as PD-L1 and MHC class II on tumor cells, which could inhibit T cells via PD-1 and LAG-3, respectively. Both high tumor IFN signaling in the tumor microenvironment and high levels of monocytic MDSCs in the circulation are associated with lower CAR T-cell expansion and a lower rate of durable responses.
Compared with B-cell lymphomas with low IFN signaling (left), high tumor IFN signaling (right) is associated with a higher number of tumor-associated macrophages, a higher level of systemic inflammatory molecules, and increased expression of immune checkpoint ligands, such as PD-L1 and MHC class II on tumor cells, which could inhibit T cells via PD-1 and LAG-3, respectively. Both high tumor IFN signaling in the tumor microenvironment and high levels of monocytic MDSCs in the circulation are associated with lower CAR T-cell expansion and a lower rate of durable responses.
In this issue of Blood, Jain et al report that immune dysregulation mediated by tumor interferon (IFN) signaling and myeloid-derived suppressor cells (MDSCs) is associated with chimeric antigen receptor (CAR) T-cell resistance in large B-cell lymphoma (LBCL).1
Despite the unprecedented efficacy of anti-CD19 CAR T-cell therapy in relapsed or refractory LBCL, durable responses are observed in <40% of patients.2,3 Prior studies have reported that antigen escape due to CD19 loss may be one mechanism of resistance observed in ∼30% of patients with LBCL who relapse after axicabtagene ciloleucel (axi-cel) anti-CD19 CAR T-cell therapy.2,4,5 Other studies showed an association between T-cell intrinsic factors and CAR-T failure: a central memory phenotype (CCR7+CD27+) of T cells in CAR-T infusion products was associated with durable responses, whereas an exhausted phenotype, defined by increased LAG-3 and TIM-3 expression, predicted early relapse.6 Similar associations have also been described between T-cell characteristics in leukapheresis products and clinical efficacy in patients with chronic lymphocytic leukemia.7 Among clinical factors, poor performance status, high systemic inflammatory state, and high tumor burden, assessed by tumor size, total metabolic tumor volume, or serum lactate dehydrogenase, have consistently been shown to be associated with lower efficacy after CAR T-cell therapy.4,5,8 Why these clinical factors are associated with inferior outcomes has been unclear. The report by Jain and colleagues provides important insights into potential mechanisms of CAR-T resistance in such patients.
The researchers analyzed serial blood and baseline tumor samples from patients with relapsed or refractory LBCL treated with axi-cel. They observed that patients with high tumor burden had high levels of inflammatory molecules, such as ferritin, IL-6, IL-15, and TNF-α and a high ANG2/ANG1 ratio in the peripheral blood. In addition, high tumor burden was associated with higher levels of IFN signaling in the tumor. Transcriptomic analysis showed that tumor IFN and macrophage gene signatures were enriched in patients who relapsed early, whereas a T-cell gene signature was enriched in patients who achieved durable responses. Consistent with a report that tumor IFN signaling upregulates PD-L1 and major histocompatibility complex (MHC) class 2 molecules9 that could suppress T cells via PD-1 and LAG-3, respectively, immunohistochemical analysis of tumor samples showed higher expression of PD-L1 and MHC class 2 in patients exhibiting early CAR-T resistance (see figure). Among immune cell subsets in the tumor microenvironment, macrophage content correlated with IFN signaling genes and early CAR-T failure. Monocytic MDSCs in the peripheral blood were also higher at baseline in patients who relapsed early after CAR T-cell therapy. High levels of tumor IFN signaling and monocytic MDSCs were associated with lower peak CAR T-cell expansion, suggesting their potential role in immunosuppression in these patients (see figure). Collectively, the results show that baseline host immune dysregulation at the tumor site and the systemic circulation characterized by tumor IFN signaling and monocytic MDSC, respectively, are important factors associated with resistance to CAR T-cell therapy. Furthermore, they provide potential explanations for why the clinical parameters of performance status and international prognostic index are powerful prognostic variables, as the former is likely to be affected by inflammatory molecules in the systemic circulation, and the latter by both systemic inflammation and tumor burden.
Although the conclusions in Jain et al are based primarily on correlations between baseline biomarkers and clinical outcome and do not directly establish causality, the multiple lines of investigation of tumor and peripheral blood samples by proteomic and/or transcriptomic analyses provide confidence for the significance of these associations and suggest novel mechanisms for CAR-T resistance. Their study suggests that targeting immune checkpoint interactions such as PD-1/PD-L1 or LAG-3/MHC class 2 may be important in enhancing CAR-T efficacy, but this is unlikely to be sufficient, as tumor IFN signaling appears to affect multiple immune molecules such as galectin-9 and V-domain Ig suppressor of T-cell activation, as well as tumor-associated macrophages. Therefore, it will be important to identify the underlying biology that drives high tumor IFN signaling in some patients. In this regard, the observation that the tumor IFN gene signature is associated with mutations in SOCS1 and KLHL6 driver genes warrants further investigation. Identifying the upstream factors central to this pathology may lead to actionable targets to improve CAR T-cell efficacy, as well as reducing toxicity, as a proinflammatory tumor microenvironment has also been associated with severe cytokine release syndrome and neurotoxicity.10 It will also be important to understand whether the lower peak CAR T-cell expansion noted in patients with high tumor IFN signaling and high levels of monocytic MDSCs is due to impairment of T-cell function at the stage of leukapheresis or of post-CAR T-cell infusion, as this would require development of different therapeutic approaches.
Conflict-of-interest disclosure: S.S.N. served as an advisory board member or consultant for Kite a Gilead Company, Merck, Bristol-Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, Unum Therapeutics, and Bluebird Bio; received research support from Kite a Gilead Company, Bristol-Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics, Allogene Therapeutics, Precision Biosciences, and Acerta; received royalties from Takeda Pharmaceuticals; and has intellectual property related to cell therapy. P.S. declares no competing financial interests.