TO THE EDITOR:
Despite recent therapeutic advances, the outcome of patients with relapsed/refractory acute myeloid leukemia (rrAML) remains poor. Treatment options are still limited for elderly patients, and allogeneic hematopoietic cell transplantation remains the only curative treatment option for the majority of transplant-eligible patients.1,2
Chimeric antigen receptor (CAR) T-cell therapy has proven significant efficacy in B-lymphoid and plasma cell–derived neoplasias.3-7 Although on-target/off-tumor toxicity with these CAR-T therapies targeting antigens such as CD19 and B-cell maturation antigen has been clinically manageable, severe cytokine release and neurotoxicity remain a clinical challenge.8,9 The transfer of conventional CAR-T technology to AML has been hampered by the fact that potential target antigens being overexpressed on leukemic blasts are also found on normal myeloid progenitor cells generating a risk for lasting aplasia.10,11 In fact, targeting of CD123, which is expressed on leukemia cells in 80% of AML patients, by conventional CAR-T cells achieved clinically meaningful remissions but led to long-lasting myelosuppression in patients not undergoing subsequent allogeneic hematopoietic cell transplantation.12-14 This illustrates the clinical need for innovative approaches to put the power of CAR-T technology under the control of reliable and fast-acting on/off switches to avoid and/or abrogate acute and long-term side effects.
The universal chimeric antigen receptor platform (UniCAR) is a 2-component, second-generation CAR-T platform using a CD28 costimulatory domain (Figure 1A). The first component is a universal CAR-T cell with a CAR that by itself does not recognize any human surface antigen but a peptide motif included in the second component, a soluble adaptor called targeting module (TM). The TM confers specificity against the cancer antigen of choice, and because of the high flexibility of the tumor-binding domain, multiple antigens in solid tumors and hematologic malignancies can be targeted.15,16 Preclinical data show that small-sized TMs efficiently penetrate and accumulate in bone marrow and solid tumor tissue.17-19 Crucially, these TMs have a short half-life of less than 30 minutes, enabling a rapid switch-off of the UniCAR system by TM withdrawal.17,20 This may avoid acute and long-term toxicity usually associated with continuous activation of CAR-T cells.17,19,20 In addition, preclinical data suggest that lysis of cancer cells by UniCAR-T cells occurs at lower TM doses than the induction of cytokine release, which suggests an improved therapeutic window for clinical applications.15,17
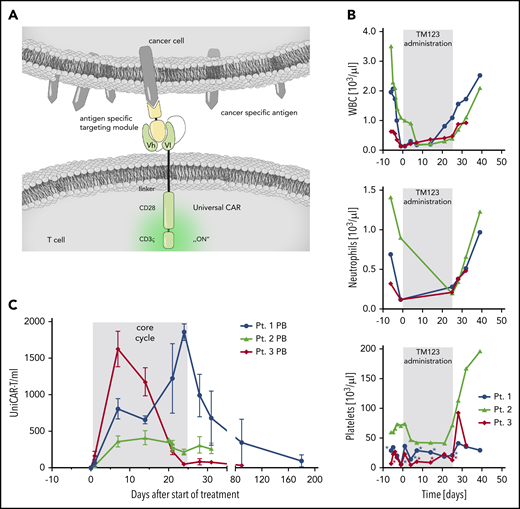
First-in-human proof-of-concept for a rapidly switchable UniCAR targeting CD123 for treatment of AML. (A) Schematic presentation of the UniCAR platform. Autologous T cells are genetically engineered to express UniCAR, which does not recognize a surface protein, and hence, UniCAR-T are inactive under physiologic conditions. A soluble adapter consisting of the UniCAR epitope (UCE) linked to a single-chain fragment variable directed against CD123 (TM123) is required for antigen-specific redirection and activation of UniCAR-T. (B) Rapid recovery of leukocytes (WBC), neutrophils, and platelets in 3 treated AML patients after stop of TM123 infusion. Asterisks indicate timing of platelet transfusions. (C) UniCAR-T-cell numbers in peripheral blood (PB) over the course of treatment. Cell number was calculated from vector copy number determined by digital droplet polymerase chain reaction, mean and standard deviation are shown.
First-in-human proof-of-concept for a rapidly switchable UniCAR targeting CD123 for treatment of AML. (A) Schematic presentation of the UniCAR platform. Autologous T cells are genetically engineered to express UniCAR, which does not recognize a surface protein, and hence, UniCAR-T are inactive under physiologic conditions. A soluble adapter consisting of the UniCAR epitope (UCE) linked to a single-chain fragment variable directed against CD123 (TM123) is required for antigen-specific redirection and activation of UniCAR-T. (B) Rapid recovery of leukocytes (WBC), neutrophils, and platelets in 3 treated AML patients after stop of TM123 infusion. Asterisks indicate timing of platelet transfusions. (C) UniCAR-T-cell numbers in peripheral blood (PB) over the course of treatment. Cell number was calculated from vector copy number determined by digital droplet polymerase chain reaction, mean and standard deviation are shown.
UniCAR-T-CD123 uses the targeting module TM123, including a single-chain fragment variable directed against the CD123 antigen, and is currently investigated in a phase 1a trial in rrAML patients with ≥20% CD123+ bone marrow blasts (NCT04230265). The primary objective of the study is to determine safety, maximum tolerated dose, and recommended phase 2 dose of this next-generation 2-component CAR-T system. Secondary objectives include objective response rate and response duration.
Here we report the results of the first 3 patients that were dosed and completed treatment in this trial. Dose finding follows a 2-dimensional matrix, using a Bayesian optimal interval (BOIN) design for drug combination trials (COMB).21,22 The clinical study was approved by the leading ethics committee (Ethikkommission an der TU Dresden) and the local ethics committees at the trial sites. Research was conducted in accordance with the Declaration of Helsinki. Dose level steps for both components were determined by a BOIN-COMB algorithm based on probability of dose-limiting toxicity as a consequence of observed adverse events. After apheresis, all patients underwent bridging therapy, which did not include leukemia-debulking chemotherapy. A lymphodepleting regimen (30 mg/m2 per day fludarabine intravenously combined with 300 mg/m2 per day cyclophosphamide intravenously for 3 days) was administered before start of treatment, which consisted of continuous intravenous infusion of TM123 from days 0 to 24. Autologous UniCAR-T cells were administered intravenously on day 1. Dosing started with 100 × 106 UniCAR-T cells and 0.5 mg TM123 per day in patient 1. Patient 2 received the same TM123 dose, but the UniCAR-T dose was increased to 250 × 106 cells. Subsequently, patient 3 received the same cell dose as patient 2 but a higher TM123 dose (1 mg/day).
To date, 8 heavily pretreated rrAML patients have been screened, 2 patients failed screening because of the absence of CD123 expression, and 2 patients died before treatment was initiated because of neutropenic infections before the initiation of lymphodepleting chemotherapy. UniCAR-T manufacturing was successful in all 5 patients undergoing apheresis. Thus far, treatment has been completed for 3 patients, with 1 patient (patient 2) receiving a second cycle of TM123. Treatment of patient 4 is ongoing with planned UniCAR-T and TM123 doses of 500 × 106 cells and 1 mg/day, respectively. Patient characteristics and initial safety and efficacy results for the first 3 patients treated are depicted in Table 1. Treatment proved to be tolerable with encouraging signs of efficacy. Myelosuppression was observed starting after lymphodepletion but immediately recovered after withdrawal of TM123 on day 24 in all patients, providing evidence for a rapid off-switch of UniCAR after stop of TM123 administration (Figure 1B). Half-life of TM123 was preclinically determined to be 0.45 hours,17 and clinical pharmacokinetic data from the first 3 patients confirmed the rapid elimination, although plasma levels were below the limit of quantification of the applied assay.
No dose-limiting toxicities were observed to date, and adverse events were generally mild (Table 1). Grade 1 cytokine release syndrome (fever) was observed in 2 patients but subsided within 48 hours after use of antipyretics. No side effect–related treatment interruption of TM123 has been necessary during cycle 1. UniCAR-T cells expanded in all patients comparable to data reported for conventional CD123 CAR-T products13 and were still detectable up to at least 6 months after administration (Figure 1C).
To date, all patients treated have shown a clinical response, with 1 patient showing a partial remission and 2 patients showing complete remission with incomplete hematologic recovery. In patient 2, leukemia is still under control 100 days after UniCAR administration; in patient 3, leukemia regrowth was detected after 1 month, and the patient is currently undergoing a second TM123 cycle, because UniCAR-T cells are still present in peripheral blood. Measurable residual disease analysis was not included per study protocol for these initial patient cohorts.
These initial clinical results of UniCAR-T-CD123 represent, to our understanding, evidence for a well-tolerated rapidly switchable CAR-T product. The efficacy seen thus far, even at the lowest dose level, is encouraging. Although the number of patients treated thus far is limited, the data obtained provide clinical proof-of-concept for the opportunity to abrogate side effects by withdrawal of TM123. Enrollment into the phase 1A study is ongoing.
For original data, please contact the corresponding author.
Acknowledgments
The authors thank Jana Hase, Antje Schubert, and Martin Lorkowski for coordinating the clinical trial and Julia Riewaldt, Cordula Gründer, Kristin Franke, Maria Schreiber, Simon Loff, Johannes Spehr, and Sonja Schallenberg for establishing analytical methods and/or UniCAR manufacturing protocol and coordinating laboratory analyses.
This study was supported in part by funding from the German Federal Ministry of Education and Research (01EK1513A).
Authorship
Contribution: M.W., A.E., M.-E.G., R.C.B., J.K., M.P., G.E., and M.C. designed the study; M.W., S.K., A.E., J.M.M., M.v.B., and M.C. collected data; and M.W., S.K., A.E., M.-E.G., R.C.B., C.K., J.K., M.P., M.B., H.E., G.E., and M.C. interpreted data.
Conflict-of-interest disclosure: A.E., J.K., M.P., G.E., and M.C. are employed by and/or hold shares of GEMoaB GmbH. C.K. is employed by CMT Cellex Manufacturing Transports and Logistics GmbH. M.W. and H.E. received honoraria from GEMoaB GmbH for their advice related to this study. The remaining authors declare no competing financial interests.
Correspondence: Gerhard Ehninger, GEMoaB GmbH, Tatzberg 47, 01307 Dresden, Germany; e-mail: g.ehninger@gemoab.com.