In this issue of Blood, Herishanu et al report on the efficacy of the BNT162b2 messenger RNA (mRNA) COVID-19 vaccine in untreated and treated patients with chronic lymphocytic leukemia (CLL).1 Their findings show low response rates to vaccination in CLL, similar to previous reports with other vaccines.
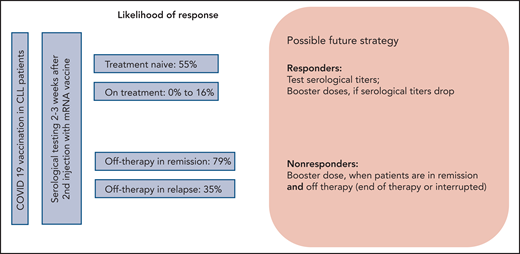
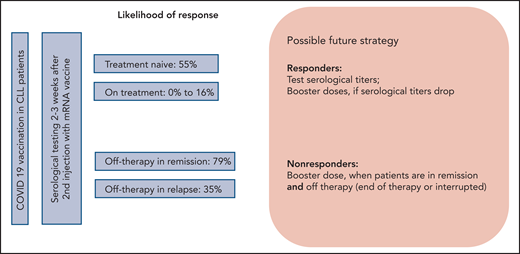
After the approval of COVID-19 vaccines, patients with CLL have been coming to their hematologist’s office with urgent questions about vaccination in their situation. The most burning questions include the likelihood of response to the COVID-19 vaccine, the optimal time to receive the vaccine, and the possible side effects (see figure). The paper by Herishanu et al is the first paper addressing these questions in a prospective study of the efficacy and tolerance of COVID-19 vaccination in a well-characterized group of patients with CLL receiving the BNT162b2 vaccine through the national Israeli vaccination program.
Studies on patients with CLL receiving antibacterial2 or antiviral3,4 vaccines have shown that the serological response to vaccination is impaired in patients with CLL because of their B-cell defects and frequent hypogammaglobulinemia. Response rates to vaccination to other viral and bacterial pathogens were a meager 8% to 59% of patients with CLL.2,3 Patients undergoing treatment, including targeted treatment with BTK inhibitors, were reported to show even lower serological response rates.2–4 With the international COVID-19 vaccinationprograms, the urgent question of howpatients with CLL were responding (or not responding) to these vaccines needed to be addressed.5
Herishanu et al included in their prospective trial, which was conducted under the auspices of the European Research Initiative on CLL, 58 treatment-naive patients with CLL, 75 actively treated patients, and 34 patients currently off treatment, with 24 of these 34 patients still in remission. Antibody response against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), predominantly immunoglobulin G (IgG) antibody, was detected in only 39% of the patients with CLL. A sex- and aged-matched analysis of 52 patients with CLL and 52 matched controls showed a 52% vs 100% response rate in patients with CLL compared with the healthy individuals. These results confirmed that there was a significantly lower serological response rate in patients with CLL. With respect to the time point of vaccination during the course of CLL, subgroup analyses showed that actively treated patients had the lowest response rates of only 16%, whereas patients who had received prior treatment and were actually in remission had the highest response rates of 79%. Completion of treatment >12 months ago was associated with even higher response rates (94%). Although no difference in response to vaccination was observed between patients receiving BTK inhibitors compared with Bcl2 inhibitor venetoclax (16.0% vs 13.6%), no response was observed in all patients who received anti-CD20 antibodies in combination with targeted agents or chemotherapy within the last 12 months. Treatment-naive patients had a 55% response rate. Type and severity of adverse events to first and second administration of the vaccine were reported in a similar range or even less frequently in comparison with healthy individuals in the approval trial for BNT162b2.6
The results reported here confirm previously published data on low serological response rates in general to vaccines2–4 and, specifically, low detectable titers of anti–SARS-CoV-2 antibodies after COVID-19 in patients with CLL.7 In addition to that, the information on response rates to vaccination for the different subgroups included in this analysis is extremely relevant for daily practice.
First, the recommendation that treatment-naive patients with CLL should receive all relevant vaccinations particularly before starting front-line therapy8 also appears to be true for COVID-19 vaccines with a response rate of >50% in that group. With respect to the good tolerance of the vaccines as shown in this trial, treatment-naive patients with CLL should therefore not postpone vaccination except due to limitation of access. Second, if patients do require treatment, the choice of therapy seems not to affect the response to vaccination on therapy, because responses across different targeted and nontargeted treatments are low as long as patients are on treatment. However, the choice of therapy likely has an impact on an optimal time point of vaccination or possible booster dose of vaccine, which, as shown here, is when the patient has stopped treatment and is in good remission.
Although some treatment options are administered as a time-limited regimen, some others, as continuous treatment with BTK inhibitors, are not. Although improvements of IgA levels during ibrutinib therapy have been observed, IgG and IgM usually remain stable, and polyclonal B lymphocytes remain low.9 Hence, it is an open question of how long BTK inhibitor or other continuously administered therapy in CLL should be interrupted, in order to achieve a better serological response rate to COVID-19 vaccines.
The paper by Herishanu et al has answered the first urgent questions. However, it also brings up more questions regarding the efficacy and optimal time point of possible booster doses of vaccines. In addition to that, data on T-cell responses following COVID-19 vaccination in patients with CLL are needed to help craft better patient protection against SARS-CoV-2, because not only humoral response but also T-cell responses play a major role in immunity.10
Following these first data from a prospective trial, more data on larger cohorts of patients with CLL as well as from different vaccines, mRNA based and vector based, are needed. COVID-19 vaccination in CLL is a particular challenge. Future strategies will have to determine how frequently patients should undergo testing for anti–SARS-CoV-2 antibodies as well as frequency and optimal time points of booster doses of vaccines or revaccinations.
Conflict-of-interest disclosure: B.E. received honoraria, participated in advisory boards, or received research grants from the following companies: Abbvie, Adaptive Biotechnologies, AstraZeneca, BeiGene, Gilead, Hexal, Janssen, MSD, Novartis, Oxford Biomedica, Pharmacyclics, and Roche. ▪