Key Points
Pomalidomide is a safe and effective therapy for severe cGVHD, including sclerotic skin manifestations.
The recommended dose is pomalidomide 0.5 mg per day orally.
Abstract
Steroid-refractory chronic graft-versus-host disease (cGVHD) is a therapeutic challenge. Sclerotic skin manifestations are especially difficult to treat. We conducted a randomized phase 2 clinical trial (#NCT01688466) to determine the safety, efficacy, and preferred dose of pomalidomide in persons with moderate to severe cGVHD unresponsive to corticosteroids and/or subsequent lines of therapy. Thirty-four subjects were randomized to receive pomalidomide 0.5 mg per day orally (n = 17; low-dose cohort) or 2 mg per day at a starting dose of 0.5 mg per day increasing to 2 mg per day over 6 weeks (n = 17; high-dose cohort). The primary endpoint was overall response rate (ORR) at 6 months according to the 2005 National Institutes of Health cGVHD Response Criteria. Thirty-two patients had severe sclerotic skin and received a median of 5 (range, 2-10) previous systemic therapies. ORR was 47% (95% confidence interval, 30-65) in the intention-to-treat analyses. All were partial responses, with no difference in ORR between the cohorts. ORR was 67% (45%-84%) in the 24 evaluable subjects at 6 months. Nine had improvement in National Institutes of Health joint/fascia scores (P = .018). Median change from the baseline in body surface area involvement of skin cGVHD was −7.5% (–10% to 35%; P = .002). The most frequent adverse events were lymphopenia, infection, and fatigue. Eight subjects in the high-dose cohort had dose decreases because of adverse events. There was 1 death in the low-dose cohort from bacterial pneumonia. Our data indicate antifibrotic effects of pomalidomide and possible association with increases in concentrations of blood regulatory T-cell and interleukin-2. Pomalidomide 0.5 mg per day is a safe and effective therapy for advanced corticosteroid-refractory cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) is an important cause of nonrelapse mortality (NRM) and functional disability in recipients of allogeneic hematopoietic cell transplantations.1 Standard systemic therapy for moderate to severe cGVHD is a corticosteroid with or without a calcineurin inhibitor. However, about half of the patients ultimately fail treatment with steroids.2,3 Ibrutinib is the only drug approved by the US Food and Drug Administration for the treatment of cGVHD after the failure of ≥1 systemic therapy,4 and new therapy options are needed.
Pomalidomide is an immune-modulating drug (IMiD) structurally related to thalidomide and is approved by the US Food and Drug Administration to treat multiple myeloma. Pomalidomide increases CD4+ T cells,5,6 suppresses T helper type 2 cells,5 may act directly on B-cell proliferation as the related lenalidomide,7 and stimulates interleukin-12 (IL-12) and soluble IL-2 receptor α production.8 Preclinical mouse models have shown that pomalidomide prevents progression of and improves skin fibrosis from bleomycin-induced injury.9
Thalidomide has been reported to be an effective therapy for severe cGVHD in several small series.10-15 However, the minimum effective dose is 200 mg per day, which is poorly tolerated and associated with sedation, constipation, and neuropathy, and is therefore rarely used.12
Pomalidomide is a more potent IMiD than thalidomide, with less bone marrow toxicity than lenalidomide.16 A phase 1/2 clinical trial reported that pomalidomide could be effective in advanced cGVHD and well tolerated at doses ≤2 mg per day.17 Involved tissues and organs with documented response include the skin, mouth, eyes, and gastrointestinal tract. We designed a phase 2 trial to test the safety and efficacy of pomalidomide, 0.5 and 2 mg per day, in subjects with moderate to severe cGVHD who failed previous therapies.
Methods
Eligibility
Subjects were 18 to 75 years of age with moderate to severe cGVHD (classic and overlap) per National Institutes of Health (NIH) criteria.18 Subjects were enrolled in a National Cancer Institute Institutional Review Board–approved treatment protocol (#NCT01688466, conducted in accordance with the Declaration of Helsinki), treated, and followed up as outpatients at the NIH Clinical Center. Subjects had to be in complete remission of their cancer and have cGVHD deemed unresponsive or progressive on high-dose corticosteroids based on an extensive review of patient history and medical records at the time of study entry (progression in at least one organ19 while on an average dose of ≥0.5 mg/kg per day of prednisone for ≥8 weeks and/or subsequent systemic therapies). Subjects had to be on a stable or decreasing dose of corticosteroids and/or any other concurrent systemic immunosuppression in the 4 weeks before study entry. Extracorporeal photopheresis had to be stopped ≥4 weeks before study entry. Additional inclusion criteria were Karnofsky performance score ≥60% and agreement to adhere to methods of contraception and other fertility control measures per Celgene Risk Evaluation and Mitigation Strategy (REMS) program. Subjects with only acute GVHD, neutrophils <1 × 109/L, platelets <75 × 109/L, estimated creatinine clearance <50 mL/min/1.73 m2 (Cockcroft-Gault formula), total bilirubin >3 mg/L, transaminase >3 times the upper limit of normal (liver cGVHD per se was not an exclusion criterion), left ventricular ejection fraction <45%, active HIV-1, hepatitis B or C infection, and NIH lung score 3 were excluded.
Study design
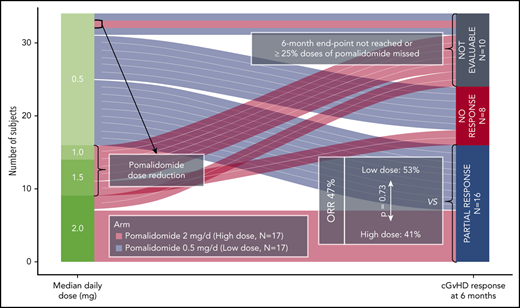
This was a randomized, open-label phase 2 clinical trial with a selection design component. Subjects were randomized to receive pomalidomide, 0.5 mg per day (low-dose [LD]) or 2 mg per day (high-dose [HD]). Subjects randomized to the HD cohort were started at 0.5 mg per day with a dose increase of 0.5 mg per day every 2 weeks (Figure 1). Pomalidomide was given orally once a day on days 1 to 28 of a 28-day cycle. In the HD cohort, dose reductions were required for grade 3 or higher nonhematologic toxicities or grade 4 or higher hematologic toxicities (supplemental Table 1, available on the Blood Web site). There was no cross-over.
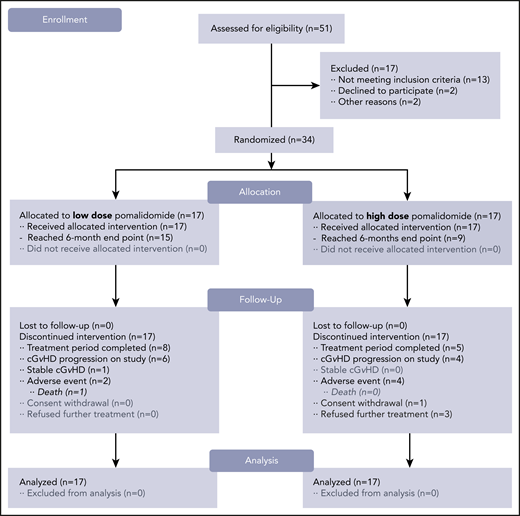
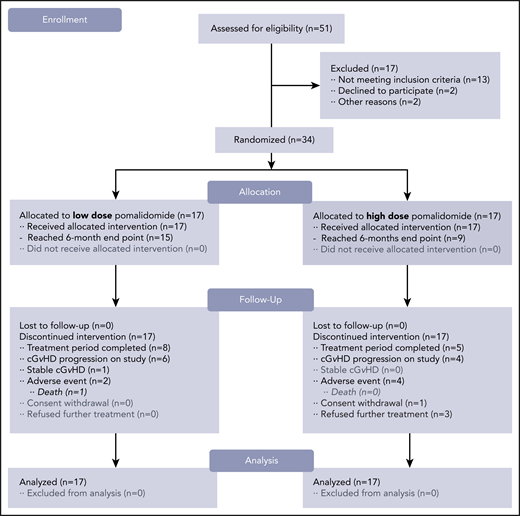
CONSORT flow diagram. Study schema. After 51 subjects were screened for the trial, a total of 34 subjects were enrolled and randomized in a 1:1 ratio. Subjects receiving pomalidomide in the LD (0.5 mg per day) cohort (n = 17) started and continued with the same daily dose. Subjects receiving pomalidomide in the HD (2 mg per day) cohort (n = 17) started with the 0.5 mg per day dose with 0.5-mg increment increases every 2 weeks until reaching the 2 mg per day dose. The 6-month ORR was the primary endpoint of the study.
CONSORT flow diagram. Study schema. After 51 subjects were screened for the trial, a total of 34 subjects were enrolled and randomized in a 1:1 ratio. Subjects receiving pomalidomide in the LD (0.5 mg per day) cohort (n = 17) started and continued with the same daily dose. Subjects receiving pomalidomide in the HD (2 mg per day) cohort (n = 17) started with the 0.5 mg per day dose with 0.5-mg increment increases every 2 weeks until reaching the 2 mg per day dose. The 6-month ORR was the primary endpoint of the study.
Concurrent topical treatments for cGVHD were permitted. The study allowed a maximum of 2 steroid pulses (0.5-2 mg/kg prednisone with taper to baseline within 3 weeks) for a cGVHD flare, defined as exacerbation of cGVHD signs and symptoms during withdrawal of immune suppression less than those at study entry. Subjects received aspirin 325 mg per day, or in some cases enoxaparin 40 mg per day, to prevent coagulation-related adverse events (AEs). Safety assessments and pregnancy testing for female subjects of child-bearing potential were performed monthly.
Response assessment
Responses were assessed every 3 months while on pomalidomide following modified 2005 NIH cGVHD Response Criteria.19 Individual organ response data collection forms (Form A) and NIH organ scoring were performed according to the 2005 NIH criteria.18,19 For individual organs, including the skin (with erythematous, movable, and nonmovable sclerosis-involved skin body surface area), eyes, mouth, gastrointestinal tract, and liver, the 2005 NIH cGVHD Response Criteria algorithms were used to determine responses.19 For the lungs, joints and fascia, health-care provider global ratings (0-10) and the criteria for progression data were collected as per the 2014 algorithms,20 including photographic range of motion.21
The primary efficacy endpoint was evaluated at 6 months. To be evaluable, subjects must have missed <25% of planned doses. Subjects with sustained partial response (PR) at 6 months and no grade 2 or higher AEs could continue receiving pomalidomide for up to 12 months. PR was defined as improvement in ≥1 organ and at least stable disease in all other organs or tissues.
Assessment of AEs
Revised National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 was used for assessment of AEs. All grade 2 or higher AEs were recorded ≤30 days after the last pomalidomide dose and monitored until return to baseline or stabilization.
Health-related quality of life and subject-reported outcomes
Subjects’ self-report questionnaires were given at baseline and every 3 months while receiving pomalidomide. These tools included the Short Form-36 Health Survey, Functional Assessment of Cancer Therapy-Bone Marrow Transplant, Human Activity Profile, and Lee symptom scale.19
Biomarker assays
Flow cytometric analyses of blood samples at baseline and at 3-month intervals assessed the relative count of regulatory CD4+ T (Treg) cells (CD3+CD4+CD25++CD127dim/–) and CD4+ and CD8+ T cells.22 Relative count of CD21–CD19+ B cells was determined within the CD20/CD19+ B-cell population (supplemental Table 2).23 Analyses were done on a Beckman Coulter Gallios flow cytometer (Beckman Coulter Life Sciences, Indianapolis, IN) and analyzed by using FlowJo version 9.9.4 software (FlowJo LLC, Ashland, OR). Frozen plasma was thawed and assessed by using enzyme-linked immunosorbent assay for IL-2 (Meso Scale Diagnostics, Rockville, MD).
Statistical analysis
With 16 subjects in each cohort, an exact binomial test would have 90% power to detect a difference between a 5% response rate and a 30% response rate using a 0.10 one-sided significance level and an exact binomial test. Three or more responses in 16 evaluable subjects would be sufficient to exclude a 5% response rate and show consistency with 30% or better response rates. As an early stopping rule for futility, if after 7 subjects were enrolled on either cohort none had responded, enrollment to that cohort would then stop. Differences between dose groups in subject baseline covariates were assessed by using the Mann-Whitney U test for continuous measures and Fisher’s exact test and the Fisher-Freeman-Halton test for categorical features. Failure-free survival was estimated according to the Kaplan-Meier method, with treatment change, NRM, and malignancy relapse as the events.24 For each event, cumulative incidence (CI) was analyzed with 2 other events as the competing risk events.25 Levels of each biomarker in evaluable subjects were compared at baseline and at 6 months by using 2-tailed Mann-Whitney U tests and Wilcoxon matched-pairs signed-rank analyses.
Pharmacokinetic variables
Plasma concentrations of pomalidomide were quantitatively measured by using a high-performance liquid chromatography assay with fluorescence detection.26 The lower limit of quantitation was 1 ng/mL. Blood samples for pomalidomide measurements were drawn predose and 2 hours postdose (±15 minutes) after the initial dose. Repeated peak and trough samples were collected 20 to 24 hours after the last dose (trough) and 2 hours after next dose (peak). Studies were repeated every 2 weeks until the 3-month evaluation and then monthly peak/trough until the 6-month evaluation. The 2 hours’ postdose was chosen as the “peak” sampling time based on published data that the time of maximum plasma concentration ranged from 1.5 to 4 hours.8
Follow-up
Subjects were followed up for 24 months after starting their first dose of pomalidomide monthly (every 3 months after discontinuing treatment).
Results
Subjects
Thirty-four subjects, with a median age of 48 years (range, 21-73 years), were randomized to receive pomalidomide 0.5 mg per day (n = 17) or 2 mg per day (n = 17) from February 2013 to October 2016. Twenty-two subjects were male. Sixteen were transplanted from HLA-identical related donors and 18 from unrelated donors (3 HLA-mismatched), with a peripheral blood stem cell graft in 28 and a bone marrow graft in 4. Thirty-two subjects had a severe NIH global score (Figure 2). All subjects had ≥2 involved organs with a median number of 5 (range, 2-8). Twenty-six had ≥20% body surface area involvement of deep skin sclerosis. Subjects received a median of 5 previous therapies (range, 2-10), including corticosteroids (n = 33), extracorporeal photopheresis (n = 28), sirolimus (n = 22), mycophenolate mofetil (n = 17), rituximab (n = 19), imatinib (n = 9), and IL-2 (n = 3). Subject baseline covariates were similar (P > .30) (Table 1).
Baseline NIH cGVHD organ-specific scores and global stage. The majority of subjects (97%) had skin and joint/fascia involvement, with all but one (97%) having moderate to severe skin and 74% moderate to severe joint/fascia cGVHD. Lungs, mouth, gastrointestinal (GI) tract, and liver were involved in 82%, 71%, 44%, and 38% of subjects, respectively, without any subject having severe organ-specific cGVHD in these organs. Eye involvement was present in 71% of subjects, with approximately one-third having severe ocular cGVHD. Ten of 12 female subjects had genital involvement (GYN). Distribution of organ-specific cGVHD was similar between the LD and HD cohorts.
Baseline NIH cGVHD organ-specific scores and global stage. The majority of subjects (97%) had skin and joint/fascia involvement, with all but one (97%) having moderate to severe skin and 74% moderate to severe joint/fascia cGVHD. Lungs, mouth, gastrointestinal (GI) tract, and liver were involved in 82%, 71%, 44%, and 38% of subjects, respectively, without any subject having severe organ-specific cGVHD in these organs. Eye involvement was present in 71% of subjects, with approximately one-third having severe ocular cGVHD. Ten of 12 female subjects had genital involvement (GYN). Distribution of organ-specific cGVHD was similar between the LD and HD cohorts.
Response
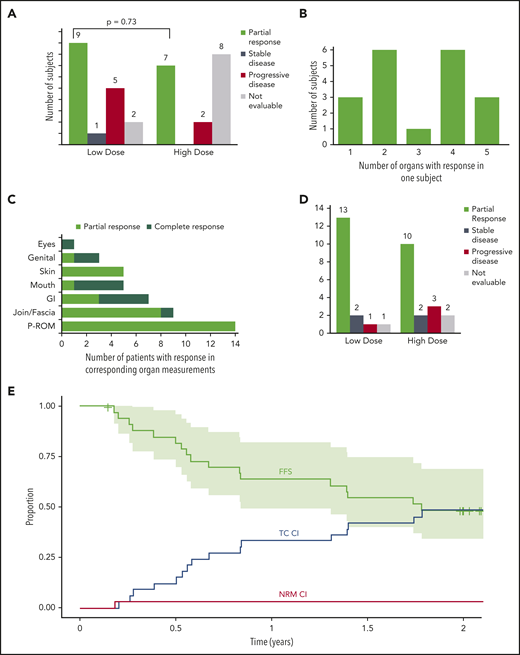
Sixteen subjects had a PR at 6 months in an intention-to-treat analysis (47% [95% confidence interval, 30-65]). The overall response rate (ORR) was 67% (45%-84%) in the 24 evaluable subjects at 6 months. The remaining 10 subjects were unevaluable at 6 months because they had early uncontrollable cGVHD progression (n = 2), withdrew consent (n = 4), developed AEs (n = 3), or received <75% of the intended dose of pomalidomide (n = 1). There was no difference in ORR between the cohorts (LD 53% [28% to 77%] vs HD 41% [18% to 67%]; P = .73) (Figure 3A). At 6 months, subjects responded in a median of 3 (1-5) organ sites (Figure 3B), and the most frequent sites with response were joint/fascia, gastrointestinal tract, mouth, and skin (Figure 3C).The best ORR at any time during on study was 68% (56%-84%) (Figure 3D). Joint/fascia NIH cGVHD scores at 6 months compared with baseline improved in 9 of the 24 evaluable subjects, worsened in 1, and no change was seen in 14 (P = .018 for overall change). These subjects also had a median decrease of 7.5% in body surface area involvement of skin cGVHD (P = .002): 13 had a decrease (median, 10% [5%-35%]), 4 had an increase (5% [5%-10%]), and 7 had no change. An example of a subject with improvement in cGVHD-related sclerosis after receiving pomalidomide is presented in supplemental Figure 1. Among the NIH cGVHD response measures, the strongest association with response was change from baseline in the 11-point health-care provider severity score (median change, −2 [–1 to −7]; P = .0003) as well as elbow (P = .0097) and wrist (P = .0093) photographic range of motion scores. During the trial, none of the subjects initiated any new active and/or passive physical therapy. After a median follow-up of 644 days (54-912 days), 6-month, 1-year, and 2-year failure-free survival was 82% (70%-96%), 64% (49%-82%), and 49% (34%-69%), respectively (Figure 3E). Two-year CI of treatment change and NRM were 48% and 3%. None of the subjects relapsed during the on-study follow-up period (24 months after the start of study drug).
Overall response at 6 months, number of subjects with multiorgan response, organ-specific response, best overall response, and failure-free survival (FFS) with cumulative incidence of FFS events. (A) Number of subjects with PR, stable disease, progressive disease, and not evaluable subjects in the LD and HD cohorts at 6 months, with a comparison of the proportion of subjects with an ORR among the 2 cohorts. (B) Number of subjects with PRs in variable involved organs. (C) Number of subjects with PRs in the specific involved organ. (D) Number of subjects with the best ORR during the study represented as PR, stable disease, progressive disease, and not evaluable in the LD and HD cohorts. (E) Kaplan-Meier FFS curve with cumulative incidence (CI) of the included events: malignancy relapse (none of the subjects experience it during the on-study follow-up), NRM, and treatment change (TC; any additional systemic treatment not previously used as the initial cGVHD treatment but excluding resumption or increment in dose of corticosteroids already used during the previous course of cGVHD treatment24 ). Curves extend to 24 months after the start of study drug (off-protocol time point). Light-green area represents 95% confidence interval. CI curves are plotted by cumulative incidence function for FFS events; for each of 3 events, the other 2 were considered as competing risk events. GI, gastrointestinal, P-ROM, photographic range of motion.
Overall response at 6 months, number of subjects with multiorgan response, organ-specific response, best overall response, and failure-free survival (FFS) with cumulative incidence of FFS events. (A) Number of subjects with PR, stable disease, progressive disease, and not evaluable subjects in the LD and HD cohorts at 6 months, with a comparison of the proportion of subjects with an ORR among the 2 cohorts. (B) Number of subjects with PRs in variable involved organs. (C) Number of subjects with PRs in the specific involved organ. (D) Number of subjects with the best ORR during the study represented as PR, stable disease, progressive disease, and not evaluable in the LD and HD cohorts. (E) Kaplan-Meier FFS curve with cumulative incidence (CI) of the included events: malignancy relapse (none of the subjects experience it during the on-study follow-up), NRM, and treatment change (TC; any additional systemic treatment not previously used as the initial cGVHD treatment but excluding resumption or increment in dose of corticosteroids already used during the previous course of cGVHD treatment24 ). Curves extend to 24 months after the start of study drug (off-protocol time point). Light-green area represents 95% confidence interval. CI curves are plotted by cumulative incidence function for FFS events; for each of 3 events, the other 2 were considered as competing risk events. GI, gastrointestinal, P-ROM, photographic range of motion.
Three subjects in each cohort received protocol-allowed corticosteroid pulses for a cGVHD flare within ≤6 months (1 pulse in 4 subjects; 2 pulses in 2 subjects) (Figure 4). Subjects requiring pulse steroids had a shorter median time from hematopoietic stem cell transplantation to study enrollment (15.5 months [11 to not available]) compared with those who did not develop a flare (43.5 [34-62]; P < .001). Five subjects subsequently discontinued the study because of cGVHD progression. One subject with a PR at 6 months continued receiving pomalidomide for another 6 months.
Response outcomes and duration of therapy in the HD and LD pomalidomide cohorts. Swimmer plot represents the duration of treatment of every individual subject in both study cohorts within the marked twelve 28-day cycles planned per protocol. The cGVHD evaluations are marked at corresponding 3-month time points, as are the time points of steroid pulses and average doses of pomalidomide per day in a cycle per patient’s diary for a corresponding cycle. F, female; M, male.
Response outcomes and duration of therapy in the HD and LD pomalidomide cohorts. Swimmer plot represents the duration of treatment of every individual subject in both study cohorts within the marked twelve 28-day cycles planned per protocol. The cGVHD evaluations are marked at corresponding 3-month time points, as are the time points of steroid pulses and average doses of pomalidomide per day in a cycle per patient’s diary for a corresponding cycle. F, female; M, male.
At the beginning of the study, 32 subjects were on concurrent treatment with a median daily dose of 20 mg (1.25-60 mg) of prednisone. Thirty-three of them were steroid refractory as defined by protocol, and one did not receive treatment with corticosteroids. At 3 months, 9 subjects received a lower dose of systemic steroids by a median of 30% (13%-50%; P = .08) and 3 subjects had an increase (1 started corticosteroids without being previously treated with it). At 6 months, 12 of 24 had a significant decrease in systemic corticosteroid dose by a median of 38% (12%-67%; P = .0005), and 11 had a stable dose. At 12 months, 1 of 13 was able to discontinue corticosteroids, and 11 had a median decrease in corticosteroid dose of 62% (17%-81%). One subject remained on 5 mg per day.
Therapy duration and median daily doses of pomalidomide
Median therapy duration for the LD and HD cohorts was 253 days (36-356 days) and 168 days (17-343 days; P = .03), respectively. Therapy was discontinued in 3 subjects in the LD cohort (2 due to AEs, 1 due to cGVHD progression) and 8 in the HD cohort (3 due to AEs, 4 consent withdrawals, and 1 due to cGVHD progression; P = .13). First subjects in the HD cohort who withdrew consent did so after resolution of cGVHD flares developed during the first cycle. The other 3 subjects withdrew due to development of AEs, including grade 3 bradycardia with grade 2 syncope during cycle 4, grade 1 neutropenia after cycle 3, and intermittent drug-induced transaminitis after cycle 3. Eight subjects in the LD cohort continued therapy for 12 months, compared with 5 subjects in the HD cohort (P = .48) (Figure 4).
Fifteen subjects in the HD cohort reached pomalidomide dose escalation to 2 mg per day after a median of 42 days (41-77 days). Subsequently, 8 subjects required dose reduction because of AEs with a median daily dose of pomalidomide less than the per-protocol intended maximal dose (median of 0.5 mg per day, n = 1; median of 1 mg per day, n = 2; median of 1.5 mg per day, n = 5).
Safety
The most commonly reported AEs were lymphocytopenia (n = 23; 8 de novo events), infection (n = 16), hypophosphatemia (n = 15), and fatigue (n = 14). Table 2 lists the most commonly recorded grade 2 or higher AEs with at least possible attribution to study treatment, although some could also be attributed to cGVHD. One female subject receiving pomalidomide 2 mg per day and aspirin 325 mg per day had a pulmonary embolism after she achieved a PR at 6 months. Rash occurred in 13 subjects (in 2 subjects considered possibly related to cGVHD flare), including 3 with bullous lesions on the lower extremities in the setting of leg edema and overlying skin contractions. Eight subjects in the LD cohort had severe AEs vs 11 in the HD cohort (P = .49). Infection was the most common severe AE in both cohorts (6 in both). There was no difference between the cohorts in frequency of AEs per subject (LD, 11 [2-18] vs HD (11 [3-17]; P = .59). Supplemental Tables 7 and 8 display AEs grade 2 or higher and severe AEs, respectively. Nine subjects in the HD cohort required dose reductions or discontinuation of therapy because of ≥1 AE vs 2 subjects in the LD cohort (P = .03). There was 1 death from bacterial pneumonia and sepsis in an LD cohort subject at 21 days on study.
Quality of life and subject-reported outcomes
Baseline median Lee symptom scale (LSS) was 41 (11-68; n = 31; 3 subjects had no baseline data). The 19 subjects evaluable for response with data at baseline and 6 months had LSS scores of 40 (11-61) and 28 (5-66; P = .06) (supplemental Figure 2A). In the 11 responders, LSS decreased from a median of 40 (11-54) to 26 (5-36; P = .035) from baseline to 6 months. There was no significant change in the 8 nonresponders (38 [17-61] vs 38 [15-66]; P = .89). Responders had improvement in LSS skin (10 [3-15] vs 4 [1-12]; P = .002) and muscle/joint–related (9 [3-15] vs 3 [0-16]; P = .027) subscales (supplemental Figure 2B-C). Subjects with improvement in cGVHD features at 6 months had an 8.7 point improvement in mean symptom severity (P < .05) and a 5-point improvement in self-reported physical health (P < .01). Subjects’ Human Activity Profile scores were unchanged compared with baseline scores. Trajectories of other patient-reported outcomes are summarized in supplemental Figures 3-6.
Long-term follow-up
Five of 13 subjects completing 12 cycles of therapy had cGVHD progression after discontinuing pomalidomide, including 3 of 8 in the LD cohort and 2 of 5 in the HD cohort. Worsening of cGVHD occurred at a median of 2 months (0.5-3 months) after discontinuing therapy. Four subjects received an increased dose or a pulse of corticosteroids. Three subjects in the LD cohort restarted pomalidomide at a dose of 0.5 mg per day, after which cGVHD stabilized in 2 subjects, who remained on study drug for 3 more months. A third subject had a PR and continued receiving pomalidomide for another year, with ongoing improvement in skin sclerosis and joint mobility. Three of 8 subjects without cGVHD progression after discontinuing pomalidomide started a new therapy; 5 received no new therapy during the 24-month follow-up.
Median interval from study entry to last contact was 24 months (2-30 months). No new deaths were reported. Three subjects developed localized squamous cell skin cancer and one basal cell skin cancer. Five subjects returned to work after completing the study.
Pharmacokinetic variables
Thirty-three subjects had pharmacokinetic data for analyses. One subject’s data were excluded because of a coeluting high-performance liquid chromatography peak in the samples that interfered with pomalidomide detection. All 33 evaluable subjects had evaluable pharmacokinetic data beyond cycle 1 (shortest cycle 2/day 1; longest cycle 7/day 1). Of the 16 subjects in the HD cohort, dose of study drug was decreased for 1 subject from 1.5 mg per day (cycle 2/day 1) to 1 mg per day by cycle 3/day 15 (off-study thereafter); for another, it was decreased from 2 mg per day (cycle 3/day 1) to 1.5 mg per day (cycle 5/day 1). Peak/trough measurements at a given dose for all subjects were averaged and plotted vs approximate estimated time of each cycle/day dose, in which time ran continuously from the start of the first dose on cycle 1/day 1. Peak concentrations increased in a dose-proportional manner (r2 = 0.99) suggesting linear pharmacokinetic variables (supplemental Figure 7; supplemental Table 9). There were no significant differences or observable trends in either peak concentrations (2 hours’ postdose), or in the predose troughs between responders and nonresponders or those subjects with a response in skin sclerosis vs nonresponders when all grouped together or stratified according to treatment arm.
Biomarkers
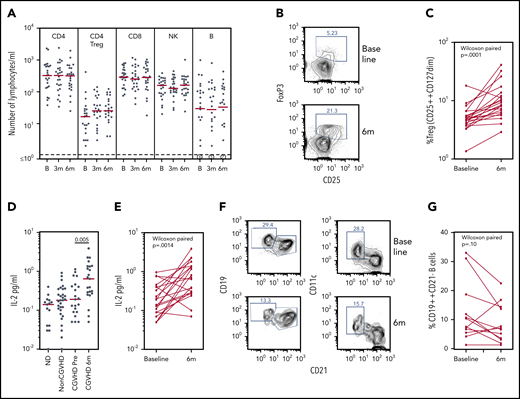
The 24 evaluable subjects were examined at baseline and 3 and 6 months for populations of T, B, and natural killer cells. Many subjects had low cell counts at baseline, particularly in the CD4 and B-cell populations (Figure 5A). There was no significant change over 6 months of treatment in the absolute numbers of CD4+ T, CD8+ T, B, or natural killer cells per microliters in the 24 evaluable subjects, in each dose cohort, or in the 16 responders (as tested by Wilcoxon matched pair signed-rank tests), with the exception that B-cell absolute numbers per microliter declined in the 16 responders (P = .013).
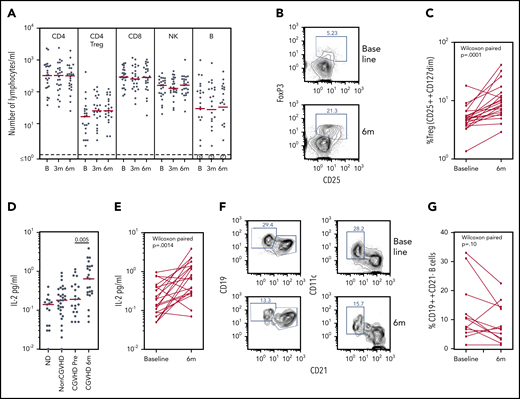
Biomarker assays at baseline and 6 months of pomalidomide treatment. (A) CD4, Treg CD4, CD8, natural killer (NK), and B lymphocyte subsets during the time course of pomalidomide treatment. Medians are marked in red. Circled numerals indicate numbers of subjects with ≤1 B cells/µL. (B) Flow cytometry of blood CD3+CD4+ Treg cells, identifying gating of FoxP3+CD25+ cells. (C) Changes in the percentage of CD25++CD127dim Treg within CD4+ T cells (Wilcoxon paired comparison of 21 subjects evaluated at both time points). (D) IL-2 levels in healthy persons, non-cGVHD patients, and pomalidomide-treated subjects at baseline and 6 months. Mann-Whitney nonparametric comparison of baseline and 6 months shown. (E) IL-2 levels in individual pomalidomide-treated subjects. Wilcoxon paired comparison of 20 subjects evaluated at both time points. (F) Flow cytometry of CD19+ B cells at baseline and 6 months identifying gating of CD19++CD21– and of CD11c+CD21– cells. (G) Changes in the percentages of CD19++CD21– B cells. Wilcoxon paired comparison of 12 subjects evaluated at both time points. B, baseline; m, months.
Biomarker assays at baseline and 6 months of pomalidomide treatment. (A) CD4, Treg CD4, CD8, natural killer (NK), and B lymphocyte subsets during the time course of pomalidomide treatment. Medians are marked in red. Circled numerals indicate numbers of subjects with ≤1 B cells/µL. (B) Flow cytometry of blood CD3+CD4+ Treg cells, identifying gating of FoxP3+CD25+ cells. (C) Changes in the percentage of CD25++CD127dim Treg within CD4+ T cells (Wilcoxon paired comparison of 21 subjects evaluated at both time points). (D) IL-2 levels in healthy persons, non-cGVHD patients, and pomalidomide-treated subjects at baseline and 6 months. Mann-Whitney nonparametric comparison of baseline and 6 months shown. (E) IL-2 levels in individual pomalidomide-treated subjects. Wilcoxon paired comparison of 20 subjects evaluated at both time points. (F) Flow cytometry of CD19+ B cells at baseline and 6 months identifying gating of CD19++CD21– and of CD11c+CD21– cells. (G) Changes in the percentages of CD19++CD21– B cells. Wilcoxon paired comparison of 12 subjects evaluated at both time points. B, baseline; m, months.
Treg populations have generally been found to be reduced in subjects with cGVHD.22,27,28 The absolute number of CD25+CD127–CD4+ Treg cells per microliter was low at baseline (median, 17 cells/µL [3-410 cells/µL]) and showed no significant change in number by 6 months (P = .17) (Figure 5A). The percentage of Treg cells within the CD4+ T cell population, however, increased under pomalidomide treatment. An increase >50% in the percent CD25+CD127– cells in the CD3+CD4+ gate was observed in 16 of 21 subjects overall, including 73% (11 of 15) of evaluable responders (P = .0001) (Figure 5B-C). Treg percentages determined in fresh whole blood were verified in 17 subjects, determined by being reassessed from cryopreserved cells as FoxP3+CD25bright CD4+ T cells.
Because IL-2 is a homeostatic factor maintaining and supporting expansion of Treg,29-31 we assessed plasma IL-2 levels by using enzyme-linked immunosorbent assays. Plasma IL-2 concentrations in subjects with cGVHD at baseline were not different from those found in normal control subjects or transplant recipients without cGVHD. IL-2 concentrations increased markedly in subjects receiving pomalidomide (P = .005) (Figure 5D), an observation confirmed by paired analyses of samples from individual subjects (P = .0014) (Figure 5E).
A subset of B cells identified as CD21–CD19bright has consistently been reported to be increased in those with treatment-refractory cGVHD.32 The CD21– B-cell subset, now further characterized as CD11c+, has been identified in cGVHD and systemic lupus erythematosus, and functionally linked to plasmablast differentiation and production of autoantibodies.33,34 Elevated percentages of CD19brightCD21– B cells (≥10%) were found in several subjects at baseline and were further characterized as CD11c+; furthermore, the percentage of CD21–CD19brightCD11c+ B cells declined in these subjects during pomalidomide treatment (Figure 5F-G). Statistical analysis of this change in a cGVHD-associated B-cell subset was confounded, however, by low absolute numbers of B cells (≤10 cells/µL) in half of the subjects at baseline and/or at 6 months. These results nonetheless suggest that monitoring B-cell subsets of interest in cGVHD may a valid addition to biomarker analysis in larger studies.
Discussion
These data indicate that pomalidomide is a safe and effective therapy for persons with severe cGVHD failing previous treatment lines, with an ORR of 47% (30%-65%) in an intention-to-treat analysis. Most subjects were on steroid therapy at the time of enrollment and were enrolled on our study to palliate bothersome symptoms. The high rate of dose reductions in the HD cohort suggests a higher toxicity without additional benefit in terms of response. Given these findings, the lower dose regimen of pomalidomide is recommended, particularly for persons with cGVHD and multiple previous therapy lines.
The most frequent organ site of response was joints/fascia, a common site of involvement in severe cGVHD.35 Although this complication is not associated with death, it is strongly associated with higher symptom burden and lower health-related quality of life.35,36 A clinically meaningful response was measured by LSS. The best clinically meaningful symptom improvements were in muscle/joint–related and skin-related LSS subscales that additionally corroborate responses in those involved organ sites.
There are few effective therapies that are known to target joint/fascia cGVHD, which is typically associated with skin sclerosis.37-40 Although none of the subjects initiated new physical therapy during the trial, many practiced physical exercises to some extent before enrollment that, if continued, could have contributed to improvements in joint/fascia scores. Because specific data on physical therapy were not documented on this study, we would recommend exploring this possible effect in the phase 3 study.
The AEs reported were similar to those seen in the pilot study of pomalidomide in cGVHD,17 including muscle cramps and fatigue. One event of pulmonary embolism occurred that could be attributed to pomalidomide but also to cGVHD because the disease itself is also associated with a higher risk of thrombosis.8,41 Due to these circumstances, it would be advisable, based on the experience with IMiDs in multiple myeloma,42 to consider thromboprophylaxis with low-molecular-weight heparin in patients with additional risk factors for a thromboembolic event. Fragility of subjects likely contributed to the 29% discontinuation rate before 6 months. Dose reductions in 44% of subjects in the HD cohort led to median daily doses of pomalidomide <2 mg per day, confounding the analyses of ORRs between the cohorts.
The difficulties in conducting clinical trials for novel therapies in cGVHD are exacerbated by the pleiotropic nature of the disorder, including inflammatory and/or fibrotic changes, various combinations of tissue and organ involvement, and different disease severity in the various involved organs. The use of biomarkers has been proposed to evaluate therapeutic efficacy but none has been validated.28 Analysis of biomarkers during pomalidomide treatment found a large increase in both plasma IL-2 levels and in the frequency of Treg cells, which can provide a protective effect against cGVHD.29,31 Pomalidomide has been shown to target cereblon, a substrate receptor of the E3 ubiquitin ligase complex CRL4CRBN.43 This complex induces degradation of the transcription factors Ikaros (IKZF1) and Aiolos (IKZF3), which are transcriptional repressors of IL-2 expression.44 Pomalidomide may therefore result in ubiquitination and degradation of IKZF1 and IKZF3, leading to a subsequent increase in endogenous IL-2 production and a consequent enhancement of Treg frequency and activity. This report of increases in IL-2 and Treg frequency supports the potential utility of pomalidomide in normalizing chronic inflammatory conditions.
One downside of pomalidomide is the potential need for steroid pulses because of early cGVHD flare. Five of the 6 subjects receiving a steroid pulse at ≤6 months did not respond to pomalidomide or were not able to continue on-study. A phase 2 trial45 of low-dose IL-2, which showed similar responses at 3 months in a somewhat less pretreated cohort (2 median lines) than ours (61% PR vs 68% in this trial) with shorter time from hematopoietic stem cell transplantation to treatment, reported no cGVHD flares during IL-2 therapy. This finding may suggest that cGVHD flares seen with pomalidomide initiation is not IL-2 driven and that a shorter time from hematopoietic stem cell transplantation to pomalidomide treatment in subjects who developed flare could represent a predictive risk factor. Lenalidomide has been found to reduce normal B-cell differentiation into pre-plasmablasts in culture,46 and it may act directly on B-cell proliferation through an additional cereblon target, the cyclin-dependent kinase inhibitor p21.7 It would be of interest to further explore pomalidomide effects on B-cell subsets in larger study populations, as well as the mechanisms by which pomalidomide mediates its antifibrotic effects.
In summary, pomalidomide is a promising new salvage therapy for persons with refractory cGVHD, overall well tolerated and effective in subjects with severe skin sclerosis and joints/fascia cGVHD manifestations. The recommended dose for a phase 3 trial of pomalidomide in cGVHD is 0.5 mg per day.
Supplemental Tables 3 to 6 contain detailed response measures at corresponding time points. Please contact the corresponding author for other original data requests (Steven Z. Pavletic; pavletis@mail.nih.gov).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank their patients and families for participating in the trial. They also thank Jackie Quan and Sharon Walter Young (Celgene Corporation) for their indispensable help in implementing the study.
Support for this research was provided by the Center for Cancer Research, National Cancer Institute, National Institutes of Health (NIH) Intramural Research Program, and Celgene Corporation through a Clinical Research Development Agreement (CRADA#02328). R.P.G. acknowledges support from the NIH Research Biomedical Research Centre funding scheme.
The views expressed do not represent the official views of the NIH or the United States Government.
Authorship
Contribution: S.Z.P., F.T.H., L.C., and D.J.V. planned the trial and experiments; F.T.H., J.J.R., and L.C. conducted experiments; S.Z.P., L.M.C., D.J.V., F.T.H., J.J.R., S.A.M., F.P., C.J.P., W.D.F., R.P.G., A.O., N.G.H., L.C., and I.P. analyzed and interpreted data; S.Z.P., L.M.C., Z.J.K., K.B., L.E.C., G.O.J., E.W.C., J.W.M., L.P.-W., and A.B. provided patient care and implemented protocol-directed procedures; L.M.C. was responsible for writing the first version of the manuscript; and all authors read and approved the final version of the typescript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Z. Pavletic, Immune Deficiency Cellular Therapy Program, Center for Cancer Research, National Cancer Institute, 10 Center Dr, Room CRC 4-3130, Bethesda, MD 20892-1907; e-mail: pavletis@mail.nih.gov.