TO THE EDITOR:
Primary testicular diffuse large B-cell lymphoma (DLBCL) shares phenotypic features with nodal activated B-cell–like (ABC) DLBCL, but originates in an immune-privileged site. Recent studies suggest that it belongs to the MCD or cluster 5 (C5) DLBCL genetic subgroup, which includes extranodal lymphomas associated with immune evasion1,2 and that often harbor an MYD88L265P mutation, a feature observed in ∼60% to 70% of cases of primary testicular DLBCL.2,3 It has a proclivity toward involvement of the central nervous system (CNS), which occurs in ∼10% to 25% of cases.4-6 Because the CNS-International Prognostic Index (CNS-IPI)7 has limited utility in testicular DLBCL, objective biomarkers assessable at diagnosis would be valuable in identifying high-risk patients.
Recurrent genomic rearrangements have been described in testicular DLBCL, as well as primary CNS lymphoma, another immune sanctuary tumor.3,8,9 However, the clinical relevance remains unknown. We used fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC) to investigate biomarker associations with clinical outcomes, including CNS risk, in testicular DLBCL.
A tissue microarray of 1.0-mm duplicate cores was constructed on all available diagnostic formalin-fixed, paraffin-embedded pretreatment DLBCL orchiectomy specimens (n = 89; diagnosis range, 1981 through 2008) that were reviewed according to the World Health Organization’s classification.10 Seven were excluded (supplemental Methods; available on the Blood Web site); thus, 82 cases were included in the present study. Patients with bilateral testicular involvement were considered to have limited stage disease in the absence of distant sites. Most patients (n = 68; 83%) received curative-intent, anthracycline-based treatment, with rituximab added for 37 patients, and 8 of them received CNS prophylaxis, with only 2 receiving IV high-dose methotrexate (Table 1).
Break-apart FISH was performed for the BCL2, BCL6, and MYC genes,11 in conjunction with a previously published FISH analysis of programmed death ligand-1 (CD274) and -2 (PDCD1LG2), as well as CIITA8 (supplemental Table 1). IHC was performed with previously defined thresholds (Table 1; supplemental Table 1).12,13 The cell of origin was determined by using the Tally and Hans algorithms.14 MYD88 mutation testing (L265P) was performed in 48 cases for which there was available tissue. Other clinical details and outcome variables are provided in the supplemental Methods. This study was approved by the University of British Columbia/BC Cancer Research Ethics Board.
Across the 5 loci surveyed via FISH, 36 (43%) cases harbored at least 1 rearrangement. BCL6 was the most frequently rearranged locus, occurring in 17 (23%) patients, and 6 (8%) harbored a PDL rearrangement (Table 1). In keeping with a prior study,3 27 of 76 cases (35.5%) had a PDL copy number alteration (PDL amplification, n = 3; 4%; PDL gains, n = 24; 31.5%; Table 1). Two (3%) were double-hit DLBCL (MYC-BCL2, n = 1; MYC-BCL6, n = 1). BCL6 and PDL rearrangements were largely mutually exclusive, with only 1 case having both rearrangements. As expected, most cases were ABC/non-germinal center B-cell (non-GCB), by either the Tally (93%) or the Hans (60%) algorithm (Table 1).14 Of interest, the frequency of MYD88L265P mutation was similar to that reported in other studies (32 of 48; 67%; Table 1)2,3 and was comparable in patients with limited- or advanced-stage (extratesticular) disease (61.5% vs 73%; P = .41), supporting that those with extratesticular involvement are most likely on the spectrum of primary testicular DLBCL.
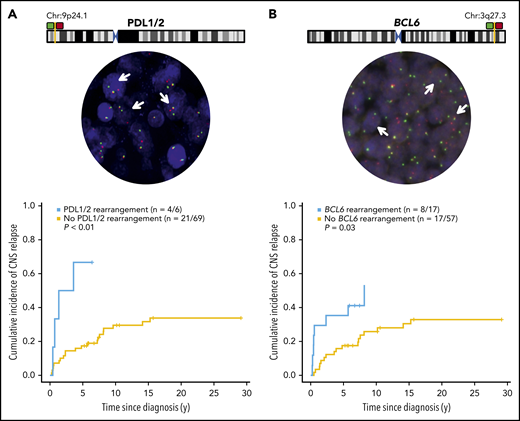
The median follow-up time for living patients was 7.1 years (range, 5.2-29). Excluding 1 patient with CNS involvement at diagnosis, the 5-year cumulative incidence of CNS relapse was 20% for the whole cohort, 22% for curative intent–treated patients, and 15% and 29% for limited and advanced-stage cases, respectively. The median time to CNS relapse was 2.96 years (0.24-15.26). The CNS-IPI was not predictive of CNS relapse risk (P = .42). However, as previously reported,5 kidney/adrenal involvement was associated with a high CNS risk, with 4 of 5 patients having CNS relapse. Interestingly, patients harboring either a PDL1/2 or BCL6 rearrangement had a significantly elevated risk of CNS relapse (PDL1/2, P < .01; BCL6, P = .03; Figure 1). Findings were similar in those treated with curative intent (supplemental Figure 1A-B). Patients with a BCL6 rearrangement were younger (P = .02) and more likely to have B symptoms (P = .05), but there was no association with stage (P = .86). There were no clinical factors associated with PDL1/2 rearrangements (results not shown). Taken together, 22 of 75 patients (29%) had either a BCL6 and/or PDL rearrangement that conferred a significantly increased risk of CNS relapse compared with those who did not harbor either rearrangement (5-year risk, 41% vs 13.5%; P < .01; supplemental Figure 2A-B). Neither gains or amplifications in PDL nor BCL6 were associated with CNS relapse (results not shown).
PDL1/2 or BCL6 chromosome maps and cumulative risk of CNS relapse by PDL or BCL6 rearrangement. The maps display relative probe positions for break-apart assays, and representative rearrangement-positive cases are depicted, with arrows denoting nuclei. Images were obtained with an Olympus BX61 microscope, original magnification ×40, at room temperature, with ARIOL software, v3.4; (Genetix). The cumulative risk of CNS relapse (using competing risk analysis) among cases with or without a PDL1- or -2 (A) or a BCL6 (B) rearrangement, as determined by FISH.
PDL1/2 or BCL6 chromosome maps and cumulative risk of CNS relapse by PDL or BCL6 rearrangement. The maps display relative probe positions for break-apart assays, and representative rearrangement-positive cases are depicted, with arrows denoting nuclei. Images were obtained with an Olympus BX61 microscope, original magnification ×40, at room temperature, with ARIOL software, v3.4; (Genetix). The cumulative risk of CNS relapse (using competing risk analysis) among cases with or without a PDL1- or -2 (A) or a BCL6 (B) rearrangement, as determined by FISH.
PDL rearrangements were associated with PDL1 (P < .001), but not PDL2 (P = .11) protein expression, strongly suggesting that PDL1 is the principal target of PDL rearrangements in testicular DLBCL (supplemental Figure 3). BCL6 rearrangement was associated with BCL6 protein expression (P = .048). Overall, 9 (11%) and 37 (45%) specimens expressed surface PDL1 and PDL2, respectively, with 6 (7%) demonstrating expression of both ligands. PDL1/2 and BCL6 protein expression were not associated with CNS recurrence (not shown).
Considering other potential CNS risk factors, there was no association with the presence of a MYD88L265P mutation (P = .51), MYC rearrangement (P = .47), double-hit mutations (P = .55), or dual-expresser status (MYC/BCL2 IHC+; P = .47). Cox multivariable analysis indicated that the presence of a PDL (HR, 8.41; 95% CI, 2.27-31.07; P = .001) or BCL6 rearrangement (HR, 4.36; (95% CI, 1.45-13.03; P < .01) were independently associated with CNS relapse. Similar results were observed when we incorporated the CNS-IPI or considered only curative-intent cases (supplemental Tables 2 and 3).
In the curative-intent cohort, PDL rearrangements were associated with an elevated risk of lymphoma relapse (P = .03); however, there was only a trend of reduced disease-specific survival (DSS; P = .09). Neither PDL1 nor PDL2 expression was associated with lymphoma relapse (PDL1, P = .59; PDL2, P = .60) or DSS (PDL1, P = .16; PDL2, P = .65). There was a trend toward reduced DSS with BCL6 rearrangement (P = .06) but not reduced risk of lymphoma relapse (P = .38), highlighting the specificity for CNS relapse, which most often occurred in isolation. No associations were observed for BCL6 protein expression (results not shown). Of interest, in contrast to nodal DLBCL, the dual-expresser phenotype was not associated with CNS relapse or DSS.
Few biomarkers have been associated with CNS relapse in DLBCL. ABC/non-GCB and dual expression of MYC and BCL2 have been associated with an elevated CNS recurrence risk15,16 ; however, this does not translate to testicular DLBCL. In contrast, the presence of either or both BCL6 and PDL rearrangements appears to confer a heightened CNS risk that is not explained by the presence of other risk factors.
The mechanisms by which BCL6 and/or PDL rearrangements enable CNS seeding remain unclear. We postulate that cases involving BCL6 and/or PDL rearrangement may be characterized by unique cross talk with the tumor microenvironment and a propensity for CNS migration as another immune privilege site. Interestingly, BCL6 rearrangements in primary CNS lymphoma have been reported to confer poor outcome.9 Future studies evaluating the expression profile may be informative regarding the tendency toward CNS relapse in testicular DLBCL, as not all CNS relapses are captured.
We confirm that most patients with testicular DLBCL harbor a MYD88 mutation, including those with advanced disease, supporting a spectrum of primary testicular DLBCL and membership in the MCD/C5 subgroup. Further, although validation studies are needed, we report for the first time, to our knowledge, that the presence of BCL6 and/or PDL rearrangements is associated with a high risk of CNS relapse, which is particularly important, given the limitations of clinical risk models in testicular DLBCL. With PD1 inhibitors under evaluation in testicular DLBCL17 (www.clinicaltrials.gov #NCT02857426), further studies should correlate these biomarkers with clinical efficacy.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Curtis Hughesman, Darko Curman, and the laboratory technologists at the Cancer Genetics Laboratory, BC Cancer, for assistance in assessing MYD88 mutation status.
This work was supported by a Canadian Institutes of Health Research (CIHR) Vanier Scholarship, an Elizabeth C. Watters Fellowship, and the University of British Columbia/PhD Training Program (D.D.W.T.); and the BC Cancer Foundation and Terry Fox Research Institute (Team Grant 1061) (C.S.).
Authorship
Contribution: D.D.W.T., C.S., and K.J.S. designed the analysis and wrote the manuscript; D.D.W.T., D.G.L., and K.J.S. performed the statistical analyses; K.L.T., G.W.S., R.D.G., and A.M. performed and reviewed the pathology and immunohistochemistry; S.B.-N. performed the FISH analyses; D.V., K.J.S., L.H.S., and J.M.C. obtained the clinical data; D.W.S. and C.S. analyzed the data; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: K.J.S. has been a consultant to and received honoraria from Bristol-Myers Squibb, Merck, Seattle Genetics, Astra Zeneca, Gilead, and Abbvie; has been a consultant to Servier; has served on the steering committee for Beigene; and has received institutional research funds from Roche. C.S. has been a consultant to and received honoraria from Seattle Genetics, Curis Inc, Bayer, and Roche and has received research funding from Bristol-Myers Squibb, Trillium Therapeutics. D.V. has been a consultant to and received honoraria from Merck, Seattle Genetics, Abbvie, Roche, Celgene, Janssen, Lundbeck, AstraZeneca, Gilead, and NanoString. D.W.S. has been a consultant to Abbvie, Celgene, and Janssen; has received research funding from Janssen, NanoString, and Roche; and has received royalties from and is a named inventor on a patent licensed to NanoString Technologies. L.H.S. has been a consultant to and received honoraria from Roche/Genentech, Abbvie, Amgen, Apobiologix, Astra Zeneca, Acerta, Celgene, Gilead, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Seattle Genetics, Teva, Takeda, TG Therapeutics, and Verastem. J.M.C. has received royalties and is a named inventor on a patent licensed to NanoString Technologies. G.W.S. has been a consultant to and received honoraria from Seattle Genetics. The remaining authors declare no competing financial interests.
Correspondence: Kerry J. Savage, BC Cancer, 600 W Tenth Ave, Vancouver, BC V5Z 4E6, Canada; e-mail: ksavage@bccancer.bc.ca.