In this issue of Blood, Greinacher and colleagues1 examine humoral responses in vaccine-induced thrombotic thrombocytopenia (VITT) and COVID-19 to determine whether these illnesses are immunologically distinct or represent a disease continuum.
At a surface level, vaccine-induced thrombotic thrombocytopenia (VITT), a recently described complication of adenoviral-based COVID-19 vaccines, and COVID-19 have much in common. Both illnesses owe their origins to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with COVID19 being caused by infection with the SARS-CoV-2 virus, whereas VITT is precipitated by vaccines encoding the SARS-CoV-2 proteins. Both conditions harness the immune system to powerful effect. Severe COVID-19 is associated with a heightened inflammatory response, characterized by increased levels of proinflammatory cytokines and markedly elevated levels of IgG and IgA antibodies to the receptor binding domain and spike protein,2,3 whereas VITT is also a byproduct of an aberrant immune response to vaccination. Mortality in both diseases is closely linked to hypercoagulability. High rates of venous thrombosis are reported in patients with severe COVID-19 infection4 and in those with VITT,5,6 with both illnesses accompanied by marked abnormalities in coagulation.5-7
However, when viewed clinically, the 2 diseases are dissimilar. COVID-19 is a viral infection, whereas VITT is not.8 COVID-19 disproportionately affects individuals with comorbidities that include older age, hypertension, and cardiovascular disease, whereas VITT occurs in healthy individuals with no discernible risk factors.8 Venous thrombosis in COVID-19 is linked to disease severity and occurs in predictable locations, such as the lower legs or pulmonary bed,9 unlike thrombosis in VITT, which often manifests in atypical locations, such as the cerebral and splanchnic vascular beds.5,6
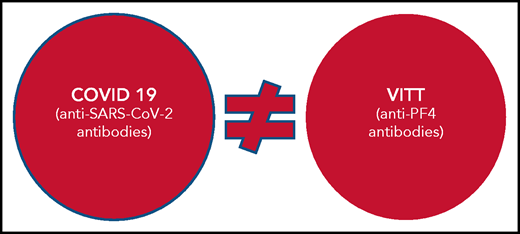
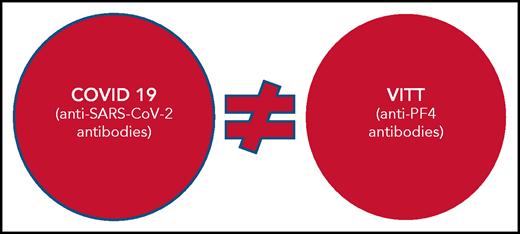
So, are these 2 conditions the same or different? This is the question Greinacher and colleagues address. Using samples from VITT and COVID-19 cohorts and affinity-purified anti-PF4 antibodies from patients with VITT, they conducted structural experiments and immunoassays and used clinical data to show that VITT is indeed its own immunologically distinct syndrome and is not related to COVID-19 or SARS-CoV-2 protective immunity.
They first provide a conceptual framework for their study by examining the structural homology of PF4 and SARS-CoV-2 proteins. Using published sequences, they identify 1 homologous motif located on both proteins (amino acid sequences 323-335 on the spike protein and 15-27 on PF4) that could provoke a convergent immune response. To identify antibodies with potential cross-reactivity, they next examined sera from patients in the VITT and COVID-19 cohorts in immunoassays, using PF4 and various spike proteins (S1, RBD-SD1, and full-length ectodomain). The experiments show robust binding of VITT sera (n = 24) to PF4 and the PF4/heparin complexes, but minimal reactivity of COVID-19 sera. As sera from both cohorts had the expected reactivity to SARS-CoV-2 proteins (related to infection and/or vaccination), the results confirm VITT antibody specificity as affinity-purified anti-P4 antibodies do not bind SARS-CoV-2 spike proteins.
Having established that anti-PF4 antibodies and anti-SARS-CoV-2 antibodies are serologically distinct, Greinacher et al next asked whether anti-PF4 antibodies could arise in COVID-19 and, if so, whether they contribute to risk of thrombosis. To investigate, they examined the seroprevalence of anti-PF4 antibodies in patients with COVID-19, with (n = 10) and without thrombosis (n = 212). In the total cohort, 8.6% (19 of 222) were positive for anti-PF4 antibodies, with only 2.7% of the total cohort expressing high levels of antibodies (optical density >1). They do not describe to what extent the seroprevalence in this study was influenced by heparin therapy, but seroprevalence was not likely to be related to heparin therapy, as the proportion of heparinized blood samples in the study (∼16%) was low. Of the seropositive patients, none were positive for heparin-dependent platelet activation, which is characteristic of antibodies in heparin-induced thrombocytopenia (HIT). Anti-PF4 antibody positivity did not correlate with severity of COVID-19 or thrombosis, generally reflecting observations in the literature that show a low incidence of HIT in COVID-19.
Several limitations of the study deserve mention. Cross-reactivity studies of anti-PF4 antibodies were limited to purified spike proteins, which could differ from the native conformation of the virus and/or differ in glycosylation. As well, antibody specificities to other SARS-CoV-2 structural proteins, including the highly immunogenic nucleocapsid protein and membrane and/or envelope proteins were not examined. Finally, the results do not further clarify the reasons why some patients develop potent anti-PF4 antibody responses.
Nonetheless, the findings reported by Greinacher et al represent an important advance in the understanding of VITT and COVID-19 for clinicians and researchers alike. For clinicians, the experiments demonstrate that VITT is its own disease entity, distinct from COVID-19 (see figure). The anti-PF4 antibodies in VITT do not appear to be a byproduct of SARS-CoV-2 protective immunity, either in the context of vaccination or COVID-19 infection. Their findings also provide some reassurance that anti-PF4 antibodies do not occur commonly in patients with COVID-19 and, if present, are generally at a low level and therefore do not confer risk of thrombosis. For researchers, the findings of nonoverlapping specificities of anti-SARS-CoV-2 and anti-PF4 antibodies suggest that the SARS-CoV-2 spike protein is unlikely to be the antigenic culprit in VITT and that additional protein candidates should be investigated.
Conflict-of-interest disclosure: G.M.A. serves as a consultant for AstraZeneca and Novartis.