Abstract
Background
Midostaurin, a multikinase inhibitor with significant activity against FLT3, was approved in combination with chemotherapy (CT) for treatment of pts with newly diagnosed (ND) FLT3-mutated AML based on the CALGB 10603/RATIFY trial (NCT00651261; Stone et al, NEJM 2017). The A2408 study (NCT03379727) confirmed the efficacy/safety of midostaurin in FLT3-mutated AML and extended the findings to older pts (≥60 y) and different CT regimens (Sierra et al, ASH 2020). As midostaurin inhibits both mutant and wild-type (WT) FLT3 (Weisberg et al, Cancer Cell 2002), the UNIFY trial (NCT03512197) was conducted to evaluate midostaurin in FLT3-mutation negative (MN) AML. Here, we report clinical outcomes and MRD results from UNIFY.
Methods
This randomized, double-blinded, multicenter, placebo (PBO)-controlled, ph 3 study evaluated midostaurin + CT in pts with ND FLT3-MN (mutant to WT signal ratio <0.05) AML. Starting Jul 2018, pts were randomized 1:1 to midostaurin or PBO and stratified by age (<60 y vs ≥60 y). Pts received midostaurin/PBO combined with daunorubicin or idarubicin + cytarabine for induction (IND; 1-2 cycles [cyc]) and with intermediate-dose cytarabine for consolidation (CONS; 3-4 cyc), followed by post-CONS (12 cyc) with midostaurin/PBO alone. Midostaurin (50 mg BID)/PBO was given from d4 or d8 (first IND) until 48 h prior to the start of the next cyc during IND/CONS and in 28-d cyc during post-CONS. The primary endpoint was EFS; secondary endpoints were OS, safety, and MRD, which was assessed by multiparameter flow cytometry (Cloos et al, J Vis Exp 2018;133:56386) using the leukemia-associated immune-phenotype (LAIP) approach. Complete panels were assessed during disease monitoring, which also allowed MRD analysis based on the different-from-normal (DfN) approach, taking into account upcoming aberrant cell populations not detected at diagnosis. Since pt remission status was not available at the time of MRD evaluation, all samples were assessed irrespective of remission.
Results
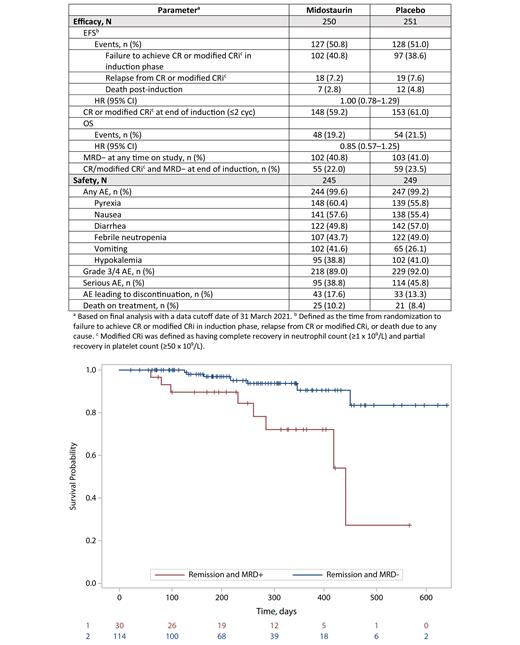
UNIFY was stopped in Sep 2019 on the recommendation of the data monitoring committee, based on an interim analysis of EFS (N = 359; HR, 1.08 [95% CI, 0.78-1.5]; data cutoff, 15 May 2019) that met the futility criterion. Final analysis occurred after the last pt discontinued and the study was closed (data cutoff, 31 Mar 2021). 501 pts were randomized to midostaurin (n = 250) or PBO (n = 251). Median age was 56 y (54% <60 y, 46% ≥60 y); 55% of pts were female and 31%/25%/35% had adverse/intermediate/favorable risk (per ELN 2017). For midostaurin and PBO, respectively, 245 and 249 pts entered IND1 and 43 and 52 entered IND2. Median exposure to study drug was 45 d for midostaurin and 50 d for PBO. Final HR for EFS was 1.00 (95% CI, 0.78-1.29); the majority of events in both treatment arms were induction failures (Table). HR for OS was 0.85 (95% CI, 0.57-1.25) in favor of midostaurin. The safety profile of midostaurin was consistent with prior reports (Table). There were no new safety signals or notable differences in toxicity between pts aged <60 y vs ≥60 y. The most common AEs leading to discontinuation were infections (7.8%) and GI disorders (4.5%) with midostaurin and infections (3.6%) with PBO. Deaths occurred on treatment in 10.2% vs 8.4% of pts receiving midostaurin vs PBO.
MRD was analyzed irrespective of treatment because no difference in MRD− rates was found between midostaurin and PBO (Table). Among randomized pts, 205 (41%) were MRD− (<0.1%) at any time on study, while MRD was undetermined (UND) in 95 (19%) due to a lack of suitable LAIP or material at diagnosis. Therefore, for the 95 pts who were MRD-UND, flow data was analyzed using the DfN approach and 70 (74%) could be assigned to either MRD− (n = 51) or MRD+ (n = 19). Among pts in CR/modified CRi (defined in Table footnote) at the end of IND, 1-y survival rates stratified by MRD were 90% for MRD− vs 72% for MRD+ (Figure).
Conclusion
Results from UNIFY are consistent with the safety/tolerability profile previously reported for midostaurin, but do not show efficacy for midostaurin in FLT3-MN AML; this suggests that the clinical effect of midostaurin in AML is primarily in the FLT3-mutated setting. Exploratory MRD analyses (to be interpreted with caution due to limited follow-up, as the study was stopped 1.2 y after first pt was randomized) suggest a trend toward longer survival for pts in remission who are MRD− at the end of IND and support the utility of assessing MRD via a combined LAIP/DfN approach.
Cloos: Takeda: Research Funding; Helsinn: Other: MRD assessments; Navigate: Patents & Royalties: Royalties for MRD analyses; DC-One: Other: MRD assessments, Research Funding; Genentech: Research Funding; Janssen: Research Funding; Novartis: Consultancy, Other: MRD assessments, Research Funding; Astellas: Speakers Bureau; Merus: Other: MRD assessments, Research Funding. Montesinos: Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Forma Therapeutics: Consultancy; Stemline/Menarini: Consultancy; Teva: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Tolero Pharmaceutical: Consultancy; Glycomimetics: Consultancy; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Agios: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astellas Pharma, Inc.: Consultancy, Honoraria, Other: Advisory board, Research Funding, Speakers Bureau. Fiedler: Daiichi Sankyo: Consultancy, Other: support for meeting attendance; Pfizer: Consultancy, Research Funding; Celgene: Consultancy; Morphosys: Consultancy; Abbvie: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Other: support for meeting attendance; Amgen: Consultancy, Other: support for meeting attendance, Patents & Royalties, Research Funding; Servier: Consultancy, Other: support for meeting attendance; Stemline: Consultancy; Novartis: Consultancy; ARIAD/Incyte: Consultancy. Müller: GSK: Consultancy; Janssen: Other: Honoraria for educational event; Celgene/BMS: Honoraria; Abbvie: Honoraria; Jazz: Honoraria; Amgen: Honoraria; Sandoz/Novartis: Honoraria; Sanofi: Honoraria; Takeda: Honoraria. Sica: Pfizer: Honoraria. Westermann: Astellas: Honoraria; Pfizer: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; BMS: Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Stem Cell Line: Consultancy, Honoraria. Döhner: Pfizer: Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Helsinn: Consultancy, Honoraria; Berlin-Chemie: Consultancy, Honoraria; Agios: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Astex: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; GEMoaB: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Ulm University Hospital: Current Employment; Novartis: Consultancy, Honoraria, Research Funding; Oxford Biomedicals: Consultancy, Honoraria. Levis: Pfizer: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen, Astellas Pharma, Daiichi-Sankyo, FujiFilm, and Menarini: Honoraria; Astellas and FujiFilm: Research Funding; Takeda: Honoraria. Ossenkoppele: Jazz: Consultancy, Honoraria; Abbvie, AGIOS, BMS/Celgene Astellas,AMGEN, Gilead,Servier,JAZZ,Servier Novartis: Consultancy, Honoraria; Agios: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Servier: Consultancy, Honoraria. Stone: Astellas: Membership on an entity's Board of Directors or advisory committees; Onconova: Consultancy; Aprea: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; Actinium: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy; Bristol Myers Squibb: Consultancy; Elevate Bio: Membership on an entity's Board of Directors or advisory committees; Foghorn Therapeutics: Consultancy; Innate: Consultancy; Janssen: Consultancy; Jazz: Consultancy; Celgene: Consultancy; Boston Pharmaceuticals: Consultancy; BerGen Bio: Membership on an entity's Board of Directors or advisory committees; Gemoab: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Agios: Consultancy, Research Funding; Macrogenics: Consultancy; Syndax: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Syntrix/ACI: Membership on an entity's Board of Directors or advisory committees; Arog: Consultancy, Research Funding. Koenen: Novartis Pharma AG: Current Employment. Bengoudifa: Novartis Pharma AG: Current Employment. Cheng: Novartis Pharmaceuticals Corporation: Current Employment. Medts: Novartis Pharma AG: Current Employment. Heidinger: Novartis Pharma AG: Current Employment. Sachs: Novartis Pharma AG: Current Employment. Sierra: Janssen: Other: Educational grant; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Other: Educational grant; Amgen: Other: Educational grant; Pfizer: Honoraria; BMS Celgene: Honoraria, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; Alexion: Other: Educational grant; Jazz Pharmaceuticals: Research Funding.