Abstract
The impact of Coronavirus disease 2019 (COVID-19) on outcomes in patients with cancer remains unclear. Acute Myeloid Leukemia (AML)/high-risk myelodysplasia (MDS) are common hematological malignancies resulting in profound immunosuppression, which is exacerbated by intensive and less-intensive chemotherapy. Importantly, venetoclax based regimens have been increasingly used during the pandemic as a strategy to reduce patient hospitalization however, there is little information concerning the impact of such regimens on COVID-19 infection rates. We therefore opened a prospective clinical study (PACE), at the start of the current pandemic in April 2020 to characterize the risk of COVID-19 infection in patients with AML/MDS-EB2 receiving intensive or non-intensive treatment, including patients treated with venetoclax-based regimens.
The primary aim was to determine the incidence of COVID-19 in patients with AML /MDS-EB2 including both, prior to study entry and during treatment until 4 weeks after the last cycle of treatment. Secondary aims were to: characterize the presentation of COVID-19; define the severity and type of both non-COVID-19 and COVID-19 infections; and undertake an exploratory analysis to quantify the incidence of COVID-19 infection in patients receiving (less-intensive) venetoclax based regimens. All analysis conducted to date has been descriptive.
211/230 recruited patients had full treatment histories available, of whom 116 patients received intensive chemotherapy and 95 low intensity regimens. 48 patients received a venetoclax-based regimen. The median age of the non-intensive treatment arm was 72 years; (range 19.1-86.5) and of the intensive arm was 59 years (range 16.1-76.1). There were more cases of secondary AML and relapsed disease in the non-intensive arm as compared to the intensive arm.
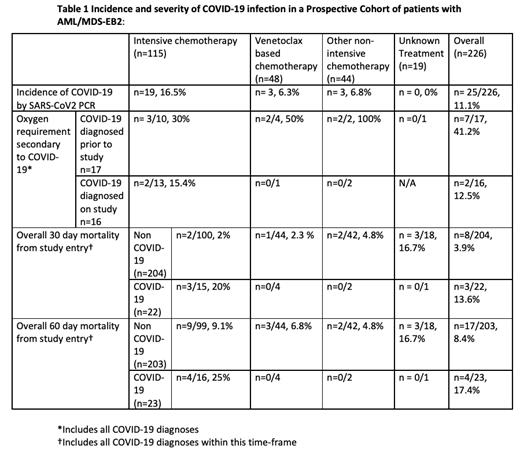
25/226 evaluable patients tested positive for COVID-19 as defined by positive SARS-CoV2 PCR test, 10 with a prior diagnosis at study entry and 15 tested positive during the study. The incidence of COVID-19 infection for patients with AML/MDS-EB2 was 11.1% (90%CI: 7.8%-15.1%) (Table). A lower proportion of patients (n=6/91 6.6%) undergoing non-intensive treatment suffered COVID-19 as compared to those undergoing more intensive chemotherapy regimens (n=19/116, 16.4%). Specifically, only 3/48 (6.3%) patients undergoing a venetoclax regimen were infected with SARS-CoV2. The most common presenting symptoms of COVID-19 in this study, regardless of the intensity of chemotherapy, was fever and cough with 6/25 patients asymptomatic. The risk of death at 30 days following study entry in patients who had prior COVID-19 infection or who contracted COVID-19 during this period was 13.6%, compared to 3.9% in the overall cohort without COVID-19 infection.
There was a lower incidence of non-COVID-19 related infections in patients receiving venetoclax-based regimens, n=43 infections in 24 (50.0%) of patients; with 313 infections in 94 (81%) of intensively treated patients. The overall occurrence of non-COVID-19 infection in the non-intensive arm was 87 infections in 50 (54.9%) patients.
Our multi-center study provides real-world estimates for the incidence and presentation of COVID-19 infection in a cohort of patients with AML/MDS-EB2, and indicates a higher risk of death at 30 days in patients with prior COVID-19 infection prior to, or during treatment. Venetoclax based, and other non-intensive, regimens, increasingly implemented during the pandemic, to minimize patient exposure and reduce usage of hospital beds, appeared to be associated with a low incidence of COVID-19. Further follow-up will be required to understand the long-term impact of this strategy. Analysis of immune responses to COVID-19 infection and vaccination is on-going.
Acknowledgments: This study was funded by Cure Leukaemia under the Trials Acceleration Program (TAP), and grants from BMS and Blood Cancer UK.
Loke: Novartis: Other: Travel; Janssen: Honoraria; Amgen: Honoraria; Pfizer: Honoraria; Daichi Sankyo: Other: Travel. Knapper: Pfizer: Consultancy, Speakers Bureau; Astellas: Ended employment in the past 24 months, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau. Khan: Abbvie: Honoraria; Astellas: Honoraria; Takeda: Honoraria; Jazz: Honoraria; Gilead: Honoraria; Novartis: Honoraria. Dillon: Amgen: Other: Research support (paid to institution); Astellas: Consultancy, Other: Educational Events , Speakers Bureau; Menarini: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Session chair (paid to institution), Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: educational events; Jazz: Other: Education events; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Research Support, Educational Events; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees. Culligan: AbbVie Ltd: Honoraria, Speakers Bureau; Celgene Ltd: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Jazz Pharma: Honoraria, Speakers Bureau; Takeda UK Ltd: Honoraria, Speakers Bureau. McMullin: Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Other: clinical trial support, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP Orphan: Research Funding, Speakers Bureau. Murthy: Abbvie: Other: support to attend educational conferences.. Craddock: Novartis Pharmaceuticals: Other: Advisory Board ; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding.