Abstract
Introduction: Venetoclax (VEN) is a BCL-2 inhibitor FDA approved in combination with azacitidine (AZA), decitabine (DEC), or low-dose cytarabine (LDAC) for newly diagnosed (ND) acute myeloid leukemia (AML) in adults aged ≥75 or those with comorbidities precluding intensive chemotherapy. We explored real-world practices and clinical outcomes of patients (pts) treated with VEN-based vs. non-VEN-based regimens in the AML Real world evidenCe (ARC) Initiative.
Methods: This multicenter chart review study includes adult pts with ND AML treated with VEN (VEN cohort) after 11 April 2016 matched to pts with non-VEN-based regimens (control cohort) after 15 May 2015 from 10 academic sites in the US and 4 in Israel. Pts were randomly selected and matched on age (<60; 60-74; ≥75) and ELN risk. Interim descriptive results (data cut: 17 May 2021) are presented; data collection is ongoing with a targeted sample of ≥500 matched pts. Response to therapy, as reported by physicians in the patient chart, hematopoietic cell transplantation (HCT) rate, and Kaplan-Meier (KM) estimates of overall survival (OS), event-free survival (EFS), and duration of response (DOR) were analyzed. Subgroups of pts aged <75 and with adverse ELN risk were analyzed separately.
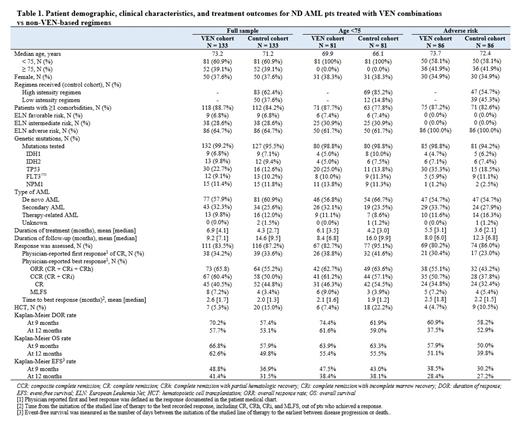
Results: Select results are shown in Table 1. 133 VEN and 133 control ND AML pts were included. Median age was 73 years (Range: 34-89) in the VEN cohort and 71 years (Range: 40-89) in the control cohort; 60.9% of matched pts were <75 (<75 subgroup; 81 VEN and 81 control). In both cohorts, 64.7% of pts had adverse ELN risk. VEN pts received VEN+AZA (106 pts, 79.7%), VEN+DEC (24 pts, 18.0%), or VEN+LDAC (3 pts, 2.3%). In the control cohort, 37.6% of pts received low-intensity regimens (largely DEC [19.5%] and AZA [14.3%]) and 62.4% received high-intensity regimens (e.g., cytarabine+daunorubicin [27.1%] and CPX-351 [15.8%]), with a higher proportion of pts on high-intensity regimens in the <75 subgroup (85.2%) and a lower proportion in the adverse-risk subgroup (54.7%). IDH1/IDH2 mutations were present in 16.5% and 15.8% of pts in the VEN and control cohorts, respectively, and NPM1 mutations were present in 11.4% and 11.8% of pts. Across subgroups, TP53 mutation was present in higher proportions of VEN pts compared to control.
The mean duration of follow-up was 9.2 months in the VEN cohort and 14.6 months in the control cohort and the mean duration of treatment was 6.9 months and 4.3 months. Composite complete remission (CCR: CR+CRi) rate was 60.4% in the VEN cohort and 50.0% in the control cohort and rate of MLFS was 7.2% and 3.4%, respectively; among the pts who achieved a response (CR, CRi, CRh, or MLFS; 81 VEN and 68 control pts), median time to best response was 1.7 months and 1.3 months, respectively. The 1-year KM DOR for patients who achieved CCR was 57.7% in the VEN cohort and 53.1% in the control cohort. After initial therapy, 5.3% of pts in the VEN cohort and 15.0% of pts in the control cohort were referred to HCT. The 1-year KM OS was 62.6% in the VEN cohort and 49.8% in the control cohort and the 1-year KM EFS was 41.4% and 31.5%.
In the <75 subgroup (81 VEN and 81 control pts), outcomes were similar between cohorts, despite a higher proportion of pts with comorbidities in the VEN cohort (87.7%) than the control cohort (77.8%); rates of CCR were 61.2% in the VEN cohort and 57.1% in the control cohort; after initial chosen therapy, 7.4% and 22.2% of pts were referred to HCT in the VEN and control cohort, respectively. The 1-year KM OS was 55.4% in the VEN cohort and 55.5% in the control cohort, and the 1-year KM EFS was 38.4% and 38.1%. In the adverse-risk subgroup, rates of CCR were 50.7% in the VEN cohort and 37.8% in the control cohort; after initial chosen therapy, 4.7% and 10.5% of pts were referred to HCT in the VEN and control cohort, respectively. The 1-year KM OS was 51.1% in the VEN cohort and 39.8% in the control cohort and the 1-year KM EFS was 28.4% and 27.2%.
Conclusions: The current analyses support real-world treatment effectiveness of VEN combinations and are consistent with data reported from clinical trials, despite the relatively short follow-up at the time of interim analyses. The outcomes, including OS at 1 year, for VEN combinations in ND AML pts appear similar to matched control pts, including the subgroup of pts <75 who largely received high-intensity regimens, and a lower proportion had comorbidities. Further analyses of matched pts are ongoing.
Garcia: AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Pfizer: Research Funding; Prelude: Research Funding; AstraZeneca: Research Funding. Wolach: Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Research Funding. Vachhani: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Talati: Jazz: Speakers Bureau; BMS: Honoraria; Pfizer: Honoraria; AbbVie: Honoraria; Astellas: Speakers Bureau. Pollyea: Karyopharm: Consultancy; Syndax: Consultancy; Amgen: Consultancy; AbbVie: Consultancy, Research Funding; Novartis: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Celgene/BMS: Consultancy; Pfizer: Consultancy; Takeda: Consultancy; Daiichi Sankyo: Consultancy; Agios: Consultancy; Glycomimetics: Other. Lai: Jazz Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Speakers Bureau; Macrogenics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Speakers Bureau; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Moshe: Astellas: Membership on an entity's Board of Directors or advisory committees, Other: Lectures; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Lectures; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Lectures. Abedin: Astellas Pharma Inc.: Research Funding; Amgen: Honoraria; Pfizer: Research Funding; Actinium: Research Funding; AltruBio: Research Funding; Agios: Honoraria; Helsinn: Research Funding. Lavie: AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Fees for lectures; BMS: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Other: Fees for lectures; Roche: Other: Fees for lectures; Novartis: Other: Fees for lectures. Zuckerman: AbbVie: Honoraria; Orgenesis Inc.: Honoraria; BioSight Ltd: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Gilead Sciences: Honoraria, Speakers Bureau; Cellect Biotechnology: Honoraria. Xavier: ADC Therapeutics: Speakers Bureau; Epizyme: Speakers Bureau; Celgene/BMS: Speakers Bureau; Seattle Genetics: Speakers Bureau; Morphosys/Incyte: Speakers Bureau; Beigene: Speakers Bureau; Acrotec: Consultancy; Genentech: Honoraria; Kite/Gilead: Honoraria; Jansen/Pharmacyclics: Honoraria; AstraZeneca: Honoraria, Speakers Bureau; Verastem: Honoraria; AbbVie: Consultancy, Speakers Bureau. Lee: AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Innate: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pin Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bui: Abbvie: Current Employment, Other: May hold equity. Svensson: AbbVie: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company. Burne: Analysis Group Inc: Current Employment, Other: Analysis Group Inc received consulting funds from AbbVie. Kye: AbbVie: Current Employment, Other: May hold equity. Maitland: Analysis Group Inc: Current Employment, Other: Analysis Group Inc received consulting funds from AbbVie. Ma: Genentech, Inc.: Current Employment, Other: May hold equity. Montez: Genentech, Inc: Current Employment, Other: May hold equity. Grunspan: AbbVie: Current Employment, Other: May hold equity. Goldberg: Aptose: Consultancy, Research Funding; Arog: Research Funding; Pfizer: Research Funding; Prelude Therapeutics: Research Funding; Aprea: Research Funding; Celularity: Research Funding; DAVA Oncology: Honoraria; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.