Abstract
Introduction:
Multiple myeloma (MM) is the cancer of plasma cells within the bone marrow (BM) and represents the second most common hematologic malignancy. Although therapeutic options have broadened over the years, the disease is challenged by frequent relapses. Relapsed/refractory MM (RRMM) often becomes non-responsive to previous lines of treatment and has significantly poorer survival outcome. Physicians are faced with a difficult task to choose a right treatment regimen. Thus, a precision medicine tool that predicts the clinical response of individual patients to therapy is greatly desired. We have previously developed a novel 3D tissue-engineered BM (3DTEBM) culture model, which is patient derived, closely recapitulates the pathophysiological conditions in the BM, and allows ex vivo proliferation of primary cells of various hematologic malignancies. In this study, we conducted a retrospective study that tests the ability of the ex vivo 3DTEBM platform to predict the clinical response in individual MM patients, to help decision-making process for RRMM. We hypothesized that the 3DTEBM will be able to predict clinical responses of RRMM patients.
Methods:
We first performed a literature search to examine the clinical efficacious concentrations (Css) for 10 MM drugs. We then experimentally determined the in vitro efficacious concentrations (IC50) of these drugs in MM cell lines, in both 2D and 3DTEBM cultures. The IC50 values were then correlated with their respective clinical Css values to evaluate how well each culture system reproduce drug efficacy. For the retrospective trial, we used viably frozen whole BM samples from 19 RRMM patients with known clinical responsiveness to the regimen they received. 3DTEBM cultures were developed for each patient with BM biopsies obtained prior to the start of clinical regimen. Cultures were treated ex vivo with the same treatment regimen each patient received clinically, at increasing concentrations (0X, 3X and 10X of Css of individual drugs). After 4 days, cultures were digested and cells were retrieved for flow cytometry analysis. Primary cells were stained Leukocyte-/CD38+ and counted against counting beads. Survival was determined as % of untreated control, and ex vivo responsiveness was analyzed by ANOVA. Finally, the clinical team correlated the ex vivo response with the clinical response for each patient.
Results:
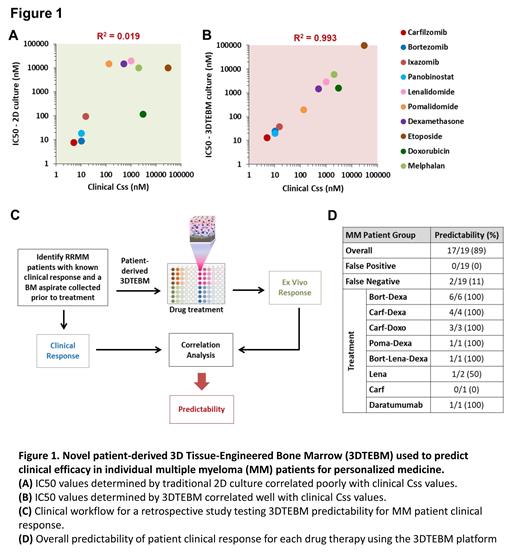
To demonstrate this discrepancy between drug efficacy in laboratory settings and clinical outcomes, we compared the in vitro IC50 to the clinical Css of 10 drugs used for the treatment of MM. We found that there was no correlation between the IC50 in classic 2D culture systems and the clinical Css (R 2=0.019) (Figure 1A). In contrast, the IC50 in the 3DTEBM directly correlated with the clinical Css (R 2=0.993) (Figure 1B). We then conducted a retrospective clinical trial to determine if the 3DTEBM platform is able to predict each patient's clinical response by recreating the same treatment regimen ex vivo (Figure 1C). The 3DTEBM was able to predict the response in 89% of the MM patient cohort across multiple treatment regimens, with no false positives (Figure 1D).
Conclusions:
Our retrospective clinical trial demonstrated that the 3DTEBM technology is a feasible platform for predicting therapeutic responses in MM with a high predictive accuracy within a clinically actionable time frame. Such platforms can provide precise clinical insight about the efficacy of different treatment plans and assist physicians to propose the best choice of therapy for their individual patients. Future prospective studies are needed to validate these significant findings by testing prospective prediction ability of 3DTEBM to improve therapy response in hematologic malignancies.
De La Puente: Cellatrix LLC: Other: Co-founder. Azab: Cellatrix LLC: Current Employment. Vij: BMS: Research Funding; Takeda: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; BMS: Honoraria; GSK: Honoraria; Oncopeptides: Honoraria; Karyopharm: Honoraria; CareDx: Honoraria; Legend: Honoraria; Biegene: Honoraria; Adaptive: Honoraria; Harpoon: Honoraria. Azab: Cellatrix, LLC: Current Employment, Current holder of individual stocks in a privately-held company.