Abstract
Introduction: Systemic mastocytosis (SM) is a rare, hematologic neoplasm driven by KIT D816V mutations in ~95% of patients. Outcomes remain poor for patients with advanced SM (AdvSM), which comprises aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). Molecular subtyping reveals a heterogeneous genetic landscape, particularly in patients with an AHN component. The KIT D816V mutation is invariably found in and considered a primary driver of the neoplastic mast cell component, while its role in the AHN component is less clear. Clinical progression (CP) can occur in the SM and/or AHN components and may be associated with outgrowth of clones with distinct mutation patterns. Avapritinib, a selective and potent inhibitor of the KIT D816V-mutant kinase, induced rapid (median time to response ~2 months), deep, and durable responses in the phase 1 EXPLORER (NCT02561988) and phase 2 PATHFINDER (NCT03580655) studies of patients with AdvSM. In these studies, responses to avapritinib treatment were observed regardless of AdvSM subtype, prior therapy, or presence of SRSF2, ASXL1, and RUNX1 (S/A/R) co-mutations that are associated with high risk. Here, we report results from an exploratory mutational analysis in patients with AdvSM enrolled in EXPLORER.
Methods: Peripheral blood (PB) and bone marrow (BM) samples were collected at baseline, during treatment, and at CP, as determined by the local investigator, for biomarker analyses and assessment of disease response to treatment. Serial samples from baseline, on study, and at end-of-treatment (when available) were evaluated centrally by droplet digital polymerase chain reaction (ddPCR) assay for detection of KIT D816V. Central next-generation sequencing (NGS; TruSight™ Myeloid Panel) of KIT and other genes mutated in myeloid malignancies was performed at baseline and on study (in a subset of patients with CP). Mutation-adjusted risk score (MARS; Jawhar et al. J Clin Oncol 2019;37:2846-2856) was used for evaluation of progression-free survival (PFS) and overall survival (OS).
Results: Among 69 patients with AdvSM in the EXPLORER study, 93% had detectable KIT D816V mutations at baseline, 3% had KIT D816Y, and 52% were positive for S/A/R co-mutations. Of patients with baseline and post-baseline assessments, 88% had a ≥50% reduction in KIT D816V variant allele fraction (VAF), with KIT D816V becoming undetectable (limit of detection <0.17%) in PB in 24 of 56 (43%) after a median of 15.4 months. Fourteen (20%) patients (SH-AHN, n=12; MCL, n=2) had documented CP on avapritinib, 6 with acute myeloid leukemia (AML), 4 with worsening AHN, 2 with SM progressions, and 2 undetermined. At the time of CP, most patients still had significant reductions in mast cell and KIT D816V clonal burden: BM mast cells (6/12 with ≥50% reduction; median 46%), PB KIT D816V VAF (10/12 with ≥50% reduction; median 83%), and serum tryptase (10/14 with ≥50% reduction; median 71%) with last assessment within 2.5 months of end of treatment. No on-target KIT resistance mutations were identified. Eight patients with CP had ≥1 S/A/R mutation at baseline. Patients with CP had a median (range) of 6 (3-9) mutations at baseline and 8 (3-12) overall, while patients without CP had 4 (1-14) mutations at baseline and 4 (1-17) overall. In patients with CP, emergent mutations occurred in NRAS, JAK2, CBL, GATA2, CUX1, SRSF2, NPM1, and SETBP1, while mutations in TET2, DNMT3A, and ASXL1 were common at baseline and retained at CP. No recurrent pattern of emergent mutations was observed. Most of these mutations had known associations with the pathogenesis of AML or myeloid AHN subtypes. MARS predicted both PFS (P=0.0126) and OS (P=0.0015), with a MARS ≥2 being associated with higher risk (Figure).
Conclusions: In EXPLORER, treatment with avapritinib in patients with AdvSM profoundly reduced KIT D816V disease burden in the majority of patients, consistent with anti-KIT D816V activity in either the SM or AHN component. Progression of the SM component was infrequent and re-emergence of KIT D816V was rare. Outcomes were more favorable in patients with low versus higher baseline MARS scores. These data suggest that avapritinib maintained control of AdvSM disease features, with low rates of CP driven primarily by progression of KIT D816V-negative AHN, and provides a rationale for future study of avapritinib in combination with AHN-directed therapy.
Deininger: Fusion Pharma, Medscape, DisperSol: Consultancy; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of a Study Management Committee, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Sangamo: Consultancy, Membership on an entity's Board of Directors or advisory committees; SPARC, DisperSol, Leukemia & Lymphoma Society: Research Funding; Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of a Study Management Committee, Research Funding; Novartis: Consultancy, Research Funding. DeAngelo: Pfizer: Consultancy; Abbvie: Research Funding; Amgen: Consultancy; GlycoMimetics: Research Funding; Shire: Consultancy; Incyte Corporation: Consultancy; Novartis: Consultancy, Research Funding; Takeda: Consultancy; Jazz: Consultancy; Forty-Seven: Consultancy; Autolus: Consultancy; Agios: Consultancy; Blueprint Medicines Corporation: Consultancy. Radia: Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Study steering group member, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Education events; EXPLORER and PATHFINDER studies: Other: Member of the Response Adjudication Committee; Cogent Biosciences Incorporated: Other: Study Steering Committee. George: Blueprint Medicines: Consultancy; Celgene: Consultancy; Incyte Corporation: Consultancy; Bristol Meyers Squibb: Consultancy. Yang: Blueprint Medicines Corporation: Current Employment, Current holder of individual stocks in a privately-held company. Sen: Blueprint Medicines Corporation: Current Employment, Current holder of individual stocks in a privately-held company. Lin: Blueprint Medicines Corporation: Current Employment, Current holder of individual stocks in a privately-held company. Mar: Blueprint Medicines Corporation: Current Employment, Current holder of individual stocks in a privately-held company. Gotlib: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Kartos: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cogent Biosciences: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Chair for the Eligibility and Central Response Review Committee, Research Funding; Allakos: Consultancy; Deciphera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
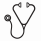 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract