Abstract
Introduction:
Outcomes of patients with large B-cell lymphoma that relapse after frontline anthracycline based chemotherapy are typically poor, with a 3-year event-free survival of approximately 30% (Gisselbrecht JCO 2010). Chimeric antigen receptor T-cell (CAR-T) therapy represents a breakthrough therapy for these patients, with an overall response rate (ORR) of 83% and a complete response (CR) rate of 58% for axicabtagene ciloleucel (axi-cel) seen in the ZUMA-1 trial, with similar rates for tisagenlecleucel in the JULIET trial (Locke Lancet Oncol 2018; Schuster NEJM 2018). Unfortunately, the majority of patients treated with CAR-T therapy experience disease progression. There is limited data evaluating the best salvage regimen for these patients. Thus, we sought to assess outcomes in large B-cell lymphoma patients with progressive disease post CAR-T cell therapy with the goal of identifying those therapies with optimal outcomes.
Methods:
A retrospective study was performed on all patients with large B-cell lymphoma undergoing leukapheresis for CAR-T therapy (tisagenlecleucel or axi-cel) at the Ohio State University from December 2017 to January 2021. Patients who died prior to CAR-T infusion were excluded from analysis. Demographics and disease characteristics as well as best response to CAR-T and date of relapse or progression following therapy were collected. First salvage therapy at relapse or progression and response to therapy were also collected, with choice of therapy driven by the treating physician. Patients were divided by salvage regimen into five groups for analysis: checkpoint inhibitor based, lenalidomide based, Bruton Tyrosine Kinase inhibitor (BTKi), chemoimmunotherapy, and other (including small molecular inhibitor, radiation, allogeneic stem cell transplant, antibody drug conjugates, and bispecific antibodies). The primary endpoint was overall survival (OS), which was calculated using Kaplan Meier Curves. Rates of CR and ORR were also assessed.
Results:
A total of 144 patients underwent leukapheresis for CAR-T cell therapy during the time period; of these patients, 13 died prior to undergoing CAR-T cell infusion and were excluded from analysis. The primary cohort included 131 patients. Median age at the time of T-cell collection was 62 years old (range 23-85) and 61% were male. The majority (50%) had germinal center (GCB) subtype, with 42% non-GCB and subtype unavailable for 8%. A small number (3%) had primary mediastinal B-cell lymphoma. The majority had high-risk disease, with 45% having primary refractory disease, 14.5% double or triple hit, and 81% Ann Arbor stage III or IV at diagnosis. Median prior lines of therapy was 3 (range 0-10). Sixty-six patients received axi-cel and 65 patients received tisagenlecleucel.
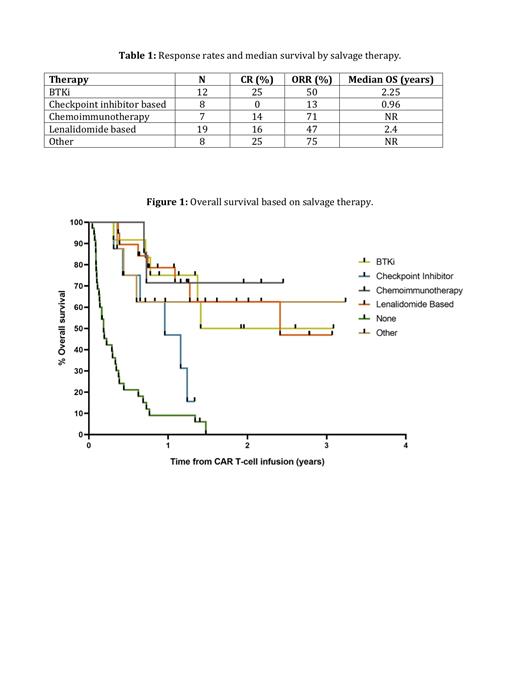
Forty percent of patients attained a CR, 18% partial response (PR), 3% stable disease (SD), 33% progressive disease (PD), and 6% of patients died prior to disease assessment. For those 76 patients that received a CR or PR to therapy, 43% relapsed post CAR-T and 57% remained in CR at last follow-up. Of those patients who relapsed or progressed post CAR-T, 69% (54/78) patients received additional therapy. The most common therapies utilized were lenalidomide based (35%), BTKi (22%), chemoimmunotherapy (13%), and checkpoint inhibitor based (15%). Other therapies represented 15% of cases and included small molecular inhibitor, radiation, allogeneic stem cell transplant, antibody drug conjugates, and bispecific antibodies (Table 1). Overall response rates and median OS for the groups were 50% and 2.25 years for BTKi, 13% and 0.96 years for checkpoint inhibitor based, 71% and not reached (NR) for chemoimmunotherapy, 47% and 2.4 years for lenalidomide based, and 75% and NR for other (Table 1; Figure 1). Median OS for those patients who did not receive any salvage therapy was 0.19 years.
Conclusion:
Consistent with prior studies, median OS following relapse post CAR-T therapy was poor, with median OS 0.19 years for patients who did not receive therapy and ranging from 0.96 years to NR for those that did. Rates of CR were highest in patients treated with BTKis. In our series, ORR rate to checkpoint inhibitors was relatively low in contrast to other recently published retrospective reports. Future prospective studies are needed to further assess the optimal therapy for patients who relapse post CAR-T therapy.
Bond: Kite/Gilead: Honoraria. Brammer: Seattle Genetics: Speakers Bureau; Kymera Therapeutics: Consultancy; Celgene: Research Funding. de Lima: Miltenyi Biotec: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Jaglowski: Novartis: Consultancy, Research Funding; Takeda: Consultancy; Juno: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; CRISPR Therapeutics: Consultancy. Kittai: Bristol-Meyers Squibb: Consultancy; Janssen: Consultancy; Abbvie: Consultancy.