Abstract
Introduction
A standard therapy for fit older adults (≥60 years) with acute myeloid leukemia (AML) being treated with curative intent consists of induction chemotherapy with cytarabine and an anthracycline followed by 1-4 cycles of shorter course post-remission therapy with cytarabine +/- anthracycline. Historically, HiDAC has been reserved for younger patients due to the high incidence of cytarabine-induced neurotoxicity, febrile neutropenia, and hospital re-admissions in older patients (Mayer et al. 1994). In select older adults (e.g., those with good-risk cytogenetic/molecular abnormalities, or requiring reinduction for persistent AML), the risk/benefit for HiDAC may favor its use. Real world evidence is lacking on the safety of full dose HiDAC (total 18 grams/cycle) in fit older adults in the 60-75 age range.
Methods
We performed a retrospective analysis of AML patients ≥60 years who received at least one cycle of HiDAC, defined as 3g/m2 every 12 hours for 6 doses, either days 1-3 or days 1, 3, 5, as post-remission therapy or primary re-induction (i.e., after failure of primary induction with 7+3 or CPX-351) between July 1, 2014 and May 28, 2021. AML risk was defined by European LeukemiaNet (ELN) guidelines. All patients had daily neurologic exams including prior to each dose of HiDAC and at the beginning of each nursing shift.
The primary endpoint was tolerability, defined as average dose per cycle, rate of dose reductions, incidence of febrile neutropenia, cerebellar toxicity, and rate of hospital re-admissions. Secondary endpoints were overall survival (OS), composite complete remission (CCR) for patients treated with HiDAC re-induction, and duration of relapse-free survival (RFS) (from day 1 of treatment) in all patients.
Results
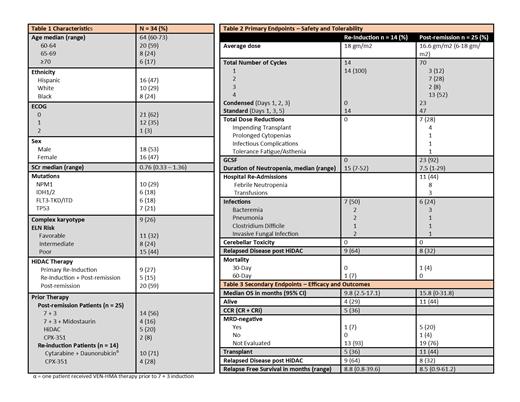
From July 2014 to May 2021, 34 patients ≥60-years-old were treated with HiDAC at our center. Median age at HiDAC administration was 64 (60-73) [Table 1]. Approximately half the patients were Hispanic and male and almost all (97%) had ECOG performance scores of 0-1. HiDAC was used as post-remission therapy (following 7+3-type induction in 80%) in 25 patients and as re-induction in 14. Eleven patients (32%) were ELN favorable risk, 8 (25%) were intermediate, and 15 (44%) were poor risk.
For post-remission therapy, the average total dose of cytarabine per cycle was 16.6 gm/m2 (6-18 gm/m2) and the median number of cycles was 4 (1-4) [Table 2]; 6 patients (24%) required dose reductions, the most common reason being upcoming allogeneic stem cell transplant (n=4). No cerebellar toxicity was observed. Duration of neutropenia was on average 15 days (7-53) in the re-induction group and 7.5 days (1-29) in the post-remission group, with 23 post-remission patients (92%) receiving granulocyte colony stimulating factors (G-CSF). Eleven hospital readmissions occurred, most commonly (73%) for febrile neutropenia. Early mortality rates were low, with one patient on the post-remission arm and one patient on the re-induction arm dying within 30 and 60 days, respectively, both due to sepsis. Median OS was 15.8 months (95% CI 0-31.8) in patients who received post-remission HiDAC, with 44% of patients still alive with a median follow up of 17.2 months (95% CI 7.2-55.8). Favorable risk patients (n=11) receiving post-remission HiDAC had median OS of 15.8 months (95% CI 0-43.1), while intermediate/poor risk patients (n=14) had median OS of 11.3 months (95% CI 9.3-13.2) [p=0.53]. Median OS was 9.8 months (95% CI 2.5-17.1) in patients who received HiDAC re-induction, with 29% of patients still alive with a median follow up of 28.6 months (95% CI 16.6-39.9) [Table 3]. Five patients (36%) achieved CCR after HiDAC re-induction, all of whom went on to receive post-remission HiDAC. Median RFS was 8.5 months for re-induction and 8.8 months for post-remission patients [Table 3].
Conclusions
HiDAC (18 grams) can be safely given to select patients over age 60. Critical to tolerability is rigorous screening of "fitness," limiting the upper age receiving HiDAC to 75, ensuring adequate renal function and euvolemia, and advances in supportive care. Encouraging survival outcomes were observed in older adults receiving post-remission or re-induction HiDAC, independent of ELN risk.
Bradley: AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sekeres: Takeda/Millenium: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Watts: Rafael Pharmaceuticals: Consultancy; Genentech: Consultancy; Bristol Myers Squibb: Consultancy; Takeda: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy; Aptevo Therapeutices: Research Funding.