Abstract
Background: Sequencing and selection of salvage regimens for acute myeloid leukemia (AML) remains unclear. Venetoclax augmentation of a hypomethylating agent (HMA) backbone has joined the salvage armamentarium following impressive performance in the front-line setting. However, little is known about the outcomes with venetoclax in relapsed and refractory (R/R) AML. Survival and toxicity data remain scarce for venetoclax-based salvage, particularly when compared to intensive reinduction. This study characterizes outcomes between venetoclax-based salvage regimens and FLAG-IDA in the R/R setting.
Patients & Methods: We retrospectively analyzed all patients with AML treated at Massey Cancer Center younger than 65 in the R/R setting with either FLAG-IDA (fludarabine, cytarabine, G-CSF, and idarubicin) or venetoclax in combination with decitabine, azacitidine, or low-dose cytarabine from June 2018 to December 2020. Baseline patient demographics and disease characteristics were obtained and recorded in RedCap. Statistical analyses using unpaired t-test with Welch's correction or the Mann-Whitney test, Fischer's exact test, and Kaplan-Meier survival analyses were computed and compared with log-rank tests using GraphPad Prism. The event for calculating the overall survival was the date of death with patients otherwise censored at the date of last contact.
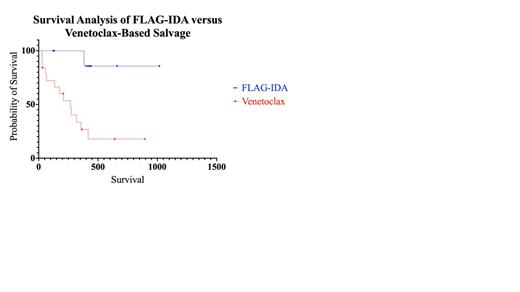
Results: Twenty-eight patients were identified meeting inclusion criteria: 19 underwent salvage with venetoclax-based regimens and 9 patients were treated with FLAG-IDA. Five patients (55.6%) in the FLAG-IDA cohort underwent salvage for refractory disease (primary induction failure), compared to only two (10.5%) in the venetoclax group, which was statistically significant (p = 0.019). The remaining patients in both cohorts were treated in the relapsed setting. There was no significant difference in sex (p = >0.999), ECOG performance status at diagnosis (p = 0.083), or Charlson Comorbidity Index (CCI) scores (p = 0.128). The median age of patients treated with FLAG-IDA was 39 (range: 24 - 62), the four most common molecular mutations in the FLAG-IDA cohort occurred at a frequency of 22.2% each: FLT3-ITD, NPM1, WT1, and biallelic CEBPA. Two patients (22.2%) had favorable cytogenetics at diagnosis, one (11.1%) had intermediate cytogenetics, five (55.6%) had adverse cytogenetics, and one was unknown. All were initially treated with a 7+3 backbone. The most common non-hematological toxicities were neutropenic fever (55.6%) and acute kidney injury (22.2%). Five (55.6%) achieved CR and one (11.1%) achieved CRi for an ORR of 66.7%. There were no deaths within 60 days of salvage. Five (55.6%) patients went on to receive allogeneic SCT. Median survival was not reached at a median follow-up of 430 days (14.1 months).
In the venetoclax-based cohort of 19 patients, the median age was 58 (range: 23 - 65), At the time of initial diagnosis, the most common molecular aberrations were TP53 (26.3%), NPM1 (15.7%), followed by FLT3-TKD, RUNX1, and TET2 (10.5% each). Two (10.5%) patients had favorable cytogenetics at initial diagnosis, two (10.5%) had intermediate, 14 (73.7%) had adverse cytogenetics, and one patient had unknown cytogenetics. Seventeen (89.5%) patients were initially treated with 7+3, one (5.3%) was treated with azacitidine, and one (5.3%) was treated with CPX-351. The most common non-hematological toxicities were infection (42.1%), neutropenic fever (31.6%), and GI toxicity (15.8%). Two (10.5%) patients achieved CR and four (21.1%) achieved CRi for an ORR of 31.6%. There were 3 deaths in 30 days and 1 death within 60 days. Four (21.1%) went on to receive allogeneic SCT after venetoclax-based salvage. The median survival was 268 days (8.81 months) and was statistically significant from the FLAG-IDA cohort (p = 0.0029).
Conclusion: Our results suggest the efficacy of FLAG-IDA compared to venetoclax-based regimens with respect to ORR, survival, toxicity, and progression to transplant in the R/R setting for presumable intensive induction candidates, as evidenced retrospectively by prior receipt of such regimens. These differences may be impacted by changes to disease biology between the relapsed and refractory settings and TP53 status. Larger prospective studies with separate analyses between the relapsed and refractory settings are needed to confirm these findings.
No relevant conflicts of interest to declare.