Abstract
Background:
Despite new therapeutic advances, acute myeloid leukemia (AML) still has poor outcomes, especially in patients with relapsed or refractory (R/R) disease with complex karyotype (CK) and/or TP53 mutation. Venetoclax (Ven), an oral BCL-2 inhibitor, in combination with DNA methyltransferase inhibitors (DNMTIs) has been approved by the FDA for treatment of newly diagnosed AML in adults who are unfit for intensive therapy with encouraging results, but the combination has been found to be less effective in patients with R/R AML.
AML cells have been shown to be sensitive to extracellular glutamine depletion or manipulation of intracellular glutamine metabolism. Asparaginase converts asparagine and glutamine to aspartate and glutamate, decreasing plasma concentrations of asparagine and glutamine, with anti-leukemia activity. We previously published that crisantaspase produced complete plasma glutamine depletion in patients without dose-limiting toxicities and was associated with anti-leukemic activity in R/R AML (Emadi et al. Cancer Chemother Pharmacol 2018). In preclinical studies, we found that Pegcrisantaspase (PegC), a long-acting crisantaspase, not only had potent single-agent anti-AML activity, but also synergized with Ven in CK-AML cell lines and primary cells in vitro and in vivo (Emadi et al. Leukemia 2021). Ven-PegC targets the mTOR-eIF4E-driven ribosomal translational protein synthesis apparatus in AML. With no standardized treatment and poor outcomes for R/R AML, there is an unmet need for effective treatment options.
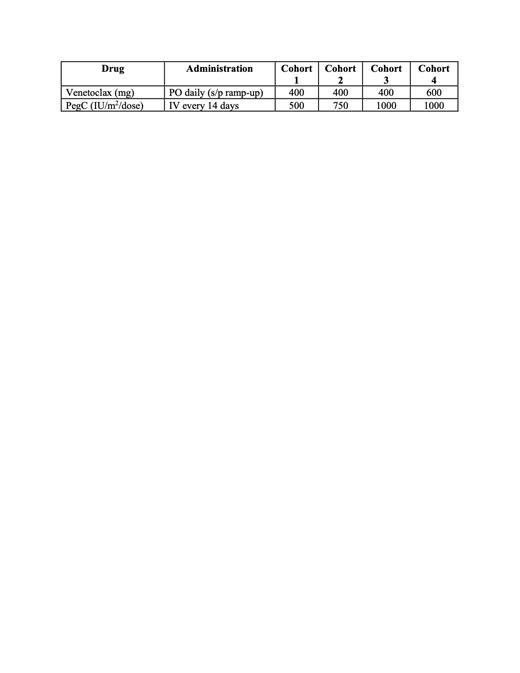
Trial Design: We present an ongoing, non-randomized, open-label Phase 1 clinical trial evaluating Ven administered orally daily in combination with PegC administered intravenously every 14 days in 28-day treatment cycles in adults patients with R/R AML. The trial consists of two phases: dose escalation (four cohorts) and dose expansion at the final recommended phase 2 doses (RP2Ds).
Adult patients with a pathologically confirmed diagnosis of AML whose disease has relapsed or is refractory to at least one line of AML therapy and with adequate organ function and no prior history of pancreatitis or ≥ Grade 3 thrombohemorrhagic events are eligible for this trial. All patients with FLT3, IDH1 or IDH2 mutation must have received at least one line of therapy with an available FLT3/IDH1/IDH2 inhibitor to be eligible for this trial. The study will include CK-AML and TP53-mutated AML.
The primary objectives of the trial are to evaluate the safety and tolerability of Ven-PegC and estimate the maximum tolerated doses (MTDs) and/or biologically active doses (e.g. RP2D) of Ven-PegC in patients with R/R AML. The primary endpoints of the trial are incidences of regimen-limiting toxicities (RLTs) and treatment-emergent adverse events (TEAEs). The secondary endpoints include the rates of complete remission (CR) and composite complete remission (CR+CRh+CRi), event-free survival, overall survival, the rate of conversion from transfusion dependence to transfusion independence, and achievement of MRD <0.02% within 2 cycles of treatment with Ven-PegC. If a patient does not achieve at least hematologic improvement within 3 cycles of treatment, the patient will be taken off study. Responding patients can continue with the assigned doses until progression.
The study uses a 3+3 design. Up to 24 subjects will be enrolled during dose escalation (in case exactly one RLT occurs in the first three patients enrolled at each of the four dose levels). Another 10 subjects will be enrolled at the final RP2D in an expansion cohort, for a total of 16 patients treated at the RP2D. The study is currently open at the University of Maryland Greenebaum Comprehensive Cancer Center. ClinicalTrials.gov Identifier is NCT04666649.
Emadi: Jazz Pharmaceuticals: Research Funding; NewLink Genetics: Research Funding; Servier: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Secura Bio.: Consultancy; KinaRx, Inc.: Membership on an entity's Board of Directors or advisory committees, Other: Co-founder.