Abstract
Background: Patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) often have a poor prognosis despite therapies using second-line chemoimmunotherapy (CIT). Achievement of complete response (CR) with second-line therapy is associated with improved long-term outcomes. Unfortunately, only 25-35% of patients achieve CR with standard CIT regimens alone. The addition of novel targeted agents such as Bruton Tyrosine Kinase inhibitors (BTKi) to second-line therapy may offer improved treatment responses given the importance of B-cell receptor (BCR) signaling in DLBCL. BTK has been shown to be essential for BCR-mediated activation of the NF- κB/Rel family of transcription factors and BCR signaling has been recognized as a key pathway in the pathogenesis of DLBCL. Moreover, NF-κB activity relies upon chronic active BCR signaling in activated B-cell-like DLBCL, which can be potentially blocked by kinase inhibitors targeting BTK. In this study we examine the feasibility and efficacy of adding the BTKi, acalabrutinib (A), to standard second-line therapy to improve disease response in patients with R/R DLBCL. Here we present initial safety and tolerability data for the ongoing study.
Study Design and Methods: This is an open-label, prospective phase II trial (NCT03736616). Cohort A is open to R/R DLBCL patients eligible for autologous hematopoietic transplantation (HCT). Cohort B is open to R/R DLBCL patients considered ineligible for autologous HCT. The primary endpoint for cohort A is to estimate the confirmed CR rate (RECIL 2017 criteria) prior to autologous HCT in patients undergoing second-line CIT. The primary endpoint for cohort B is defined as the estimate of one-year progression-free survival in patients undergoing second-line induction and maintenance acalabrutinib therapy.
Cohort A receive 2 cycles of standard RICE salvage CIT in combination with acalabrutinib, 100mg BID days 1-21 of a 21-day cycle (RICE+A). After 2 cycles of therapy, patients undergo autologous stem cell mobilization and collection. Patients then receive a 3 rd cycle of RICE in combination with acalabrutinib. PET-CT (PET3) is to be performed on day 15 of cycle 3 to assess response. Patients with CR or partial response (PR) after PET3 proceed to autologous HCT with BEAM conditioning within 28-42 days of PET3. Post-HCT CR patients receive acalabrutinib 100mg BID as maintenance therapy for 12 additional months. Protocol amendment in May 2021 allows for PET assessment (C2D15) prior to autologous stem cell collection (after cycle 3).
Cohort B receive 3 cycles of RICE+A in 21-day cycles followed by PET-CT (PET3) on day 15 of cycle 3. Patients without progressive disease at PET3 continue with acalabrutinib maintenance up to 12 additional cycles until disease progression or unacceptable toxicity. Patients demonstrating progressive disease are withdrawn from study treatment but followed for outcomes.
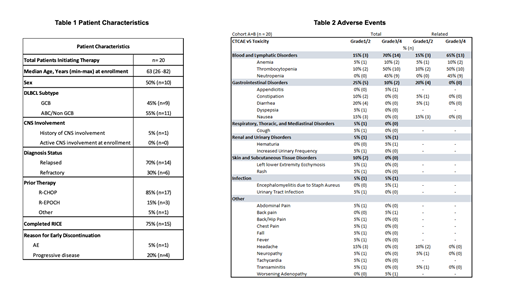
Results: Here we report initial safety and tolerability data for the ongoing study with data cutoff July 28, 2021. Twenty-two patients have been screened and 20 patients have received at least 1 cycle of RICE+A. Patient characteristics are shown in Table 1. Fifteen patients (79%) have completed 3 cycles of RICE+A. One patient (5%) discontinued due to an adverse event (AE; recurrent appendicitis), 3 patients (16%) discontinued due to progressive disease, and 1 patient is receiving ongoing RICE+A as of data cutoff. Hematologic AE have been observed in 17 patients (89%) with 74% being Grade 3/4. Amongst these, neutropenia was the most common AE occurring in 47% with all being Grade 3/4, and thrombocytopenia occurring in 32% with all being Grade 3/4. All hematologic AE recovered to baseline or grade 1 in median 7 days. Amongst non-hematologic AE, diarrhea occurred in 21% and 0% were Grade 3/4, nausea 16% with 0% Grade 3/4, and headache in 16% with 0% Grade 3/4. Discontinuation of therapy due to AE occurred in 1 patient (recurrent appendicitis) and dose reduction occurred in 1 patient (Gr 4 neutropenia). Temporary (per protocol) dose holds of A occurred in 9 patients (45%), primarily for cytopenias during concurrent RICE+A. Median duration for dose holds of A was 5.5 days.
Conclusion: RICE+A is feasible with manageable primarily hematologic AEs similar to those reported for RICE alone. Enrollment and follow up is ongoing for efficacy endpoints and further toxicity assessment.
Bensinger: BMS, Janssen, Poseida, Regeneron, Trillium: Research Funding; Amgen, BMS, Janssen, Sanofi: Speakers Bureau. Glennie: Pharmacyclics/Janssen: Speakers Bureau. Pagel: Pharmacyclics/AbbVie: Consultancy; Incyte/MorphoSys: Consultancy; MEI Pharma: Consultancy; Gilead: Consultancy; Actinium Pharmaceuticals: Consultancy; AstraZeneca: Consultancy; BeiGene: Consultancy; Kite, a Gilead Company: Consultancy; Epizyme: Consultancy. Patel: Bristol Myers Squibb: Consultancy, Speakers Bureau; Janssen: Consultancy; Genentech: Consultancy; BeiGene: Consultancy; TG Therapeutics: Consultancy, Speakers Bureau; Abbvie: Consultancy; Pharmacyclics: Consultancy; Morphosys: Consultancy; Kite Pharma: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; MEI Pharma: Consultancy; ADC Therapeutics: Consultancy; Lilly: Consultancy.
Acalabrutinib is not FDA approved for treatment of DLBCL and is discussed in the context of an ongoing clinical trial only.