Abstract
Introduction: Multiple myeloma accounts for 1% of all cancers and approximately 10% of all hematologic malignancies. With the advent of novel therapies for multiple myeloma and emerging data from randomized trials, there has been a substantial improvement and favorable outcomes in survival. Here, we report demographics and outcomes in a cohort of patients who underwent Autologous Stem Cell Transplant (ASCT) over the past year.
Methods: In this retrospective, cohort study, we assessed all MM patients who received ASCT from January 1, 2020, to January 15, 2021, at Cleveland Clinic, and followed until July 31, 2021. Baseline demographics, ECOG performance status, ISS stage, cytogenetic risk category, therapy received before ASCT, maintenance therapy, time to first relapse/progression, time to next treatment (TTNT; 2nd line of treatment onwards) with treatment response (defined per IMWG response criteria) before and after ASCT were obtained by review of electronic medical records. All patients received HDCT Melphalan 140 mg/m2 or 200 mg/m2 prior to ASCT. Continuous variables were presented as median and interquartile range, while categorical variables were presented as numbers and percentages. Categorical variables were compared using the chi-square test.
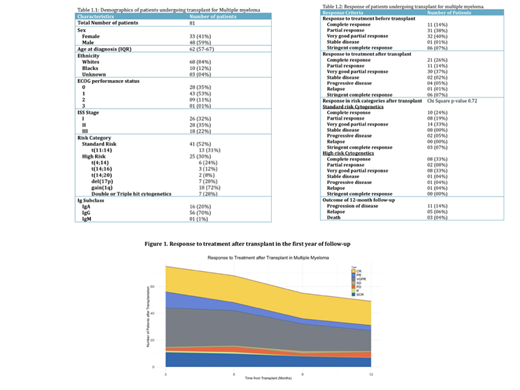
Results: Of 81 MM patients who underwent ASCT, 59% were males, 84% were white, with a median age of 62 (IQR: 57-67) at the time of diagnosis. 42 (52%) and 24 (30%) patients belonged to the standard and high-risk category. Amongst high-risk cytogenetic abnormalities, +1q was the most common (72%) followed by del(17p) (28%). Baseline characteristics of patients are included in Table 1.1. Lenalidomide, bortezomib, and dexamethasone (VRd) regimen was the most common (75%) first-line induction regimen used, followed by Daratumumab-based regimens (20%). 10 (12%) patients required second-line treatment, and 6 (7%) patients required more than 2 lines of treatments prior to transplant. The median time to transplant was 6.5 months. The overall response rate (ORR) prior to transplant was 99% (21% complete (CR), 40% very good partial (VGPR), and 38% partial (PR)). The ORR post-ASCT was 78% (CR 27%, VGPR 37%, PR 14%). There was no significant difference in response between risk categories after transplant (P=0.72). At 1-year follow-up, 10 (12%) patients had relapsed and 7 (9%) patients had progression of the disease. 3 (4%) patients died of progressive MM, one of which had progressed to plasma cell leukemia. Response to treatment before and after the ASCT are summarized in Table 1.2 and Figure 1. The time to second-line treatment among patients with relapse/progression was 7 months [IQR: 3.75-10.25].
Conclusion: Here we report the demographics and outcomes of patients with MM undergoing modern modality treatments and ASCT, at our center over the last year. The median time to transplant was 6.5 months after induction therapy, and the ORR post-ASCT was 78%. No significant difference in response was observed between high and standard risk categories. No transplant-related mortality was observed as well.
Anwer: Allogene Therapeutics: Research Funding; Janssen pharmaceutical: Honoraria, Research Funding; BMS / Celgene: Honoraria, Research Funding; GlaxoSmithKline: Research Funding.