Key Points
Immune (Ab & T) responses to BNT162b2 mRNA COVID-19 vaccine have been previously found to be impaired in patients with hematological neoplasms.
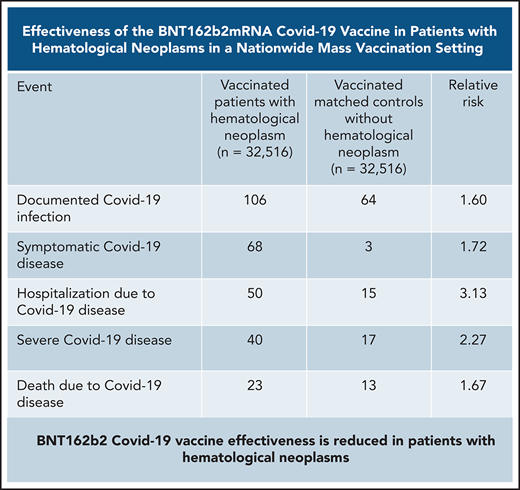
BNT162b2 COVID-19 vaccine effectiveness is reduced in patients with hematological neoplasms, especially in those receiving active therapy.
Abstract
Evidence regarding the effectiveness of COVID-19 vaccine in patients with impaired immunity is limited. Initial observations suggest a lower humoral response in these patients. We evaluated the relative effectiveness of the mRNA BNT162b2 vaccine in patients with hematological neoplasms compared with matched controls. Data on patients with hematological neoplasms after 2 vaccine doses were extracted and matched 1:1 with vaccinated controls. Subpopulation analyses focused on patients receiving therapy for hematological neoplasm, patients without treatment who were only followed, and recipients of specific treatments. The analysis focused on COVID-19 outcomes from days 7 through 43 after the second vaccine dose in these areas: documented COVID-19 infection by polymerase chain reaction; symptomatic infection; hospitalizations; severe COVID-19 disease; and COVID-19–related death. In a population of 4.7 million insured people, 32 516 patients with hematological neoplasms were identified, of whom 5017 were receiving therapy for an active disease. Vaccinated patients with hematological neoplasms, compared with vaccinated matched controls, had an increased risk of documented infections (relative risk [RR] 1.60, 95% CI 1.12-2.37); symptomatic COVID-19 (RR 1.72, 95% CI 1.05-2.85); COVID-19–related hospitalizations (RR 3.13, 95% CI 1.68-7.08); severe COVID-19 (RR 2.27, 95% CI 1.18-5.19); and COVID-19–related death (RR 1.66, 95% CI 0.72-4.47). Limiting the analysis to patients on hematological treatments showed a higher increased risk. This analysis shows that vaccinated patients with hematological neoplasms, in particular patients receiving treatment, suffer from COVID-19 outcomes more than vaccinated individuals with intact immune system. Ways to enhance COVID-19 immunity in this patient population, such as additional doses, should be explored.
Introduction
Mass vaccination campaigns against COVID-19 are being conducted worldwide. The mRNA-based vaccines were proven to be effective in randomized clinical trials, preventing COVID-19 infection in the range of 94% to 95%1,2 and in real-life settings.3 Vaccinated individuals experienced a reduced rate of all studied outcomes compared with unvaccinated controls.3
Patients with solid tumors and hematological neoplasms who suffer from impaired immunity are at particular risk, with higher morbidity and mortality.4-7 Unfortunately, data on vaccination effectiveness in this patient population are still limited.
Israel’s national policy has encouraged all patients with malignancies to be vaccinated. Thus, most patients with hematological neoplasms in Israel received 2 consecutive doses of the BNT162b2 mRNA COVID-19 vaccine (Pfizer), administered 21 days apart, as a standard of care through the national vaccination program.
Preliminary reports have suggested a low seroconversion rate in vaccinated patients with hematological neoplasms compared with healthy controls,7-15 but the reports were not powered to assess COVID-19–related outcomes. These observations raise concerns regarding the outcomes of COVID-19 exposure in such vaccinated patients. Whether the lower seroconversion rates in this patient population are associated with lower clinical effectiveness remains to be determined.
The primary aim of this study was to test the relative effectiveness of the BNT162b2 mRNA COVID-19 vaccine in patients with hematological neoplasms compared with vaccinated matched controls. The secondary aims were to study the vaccination effect in certain subgroups of patients with hematological neoplasms as well as the effect in patients receiving specific hematological treatments.
Methods
Setting
The base population for this study was the population of Clalit Health Services (CHS), the largest health care organization in Israel, which insures 4.7 million people (52% of the population).
Patient population
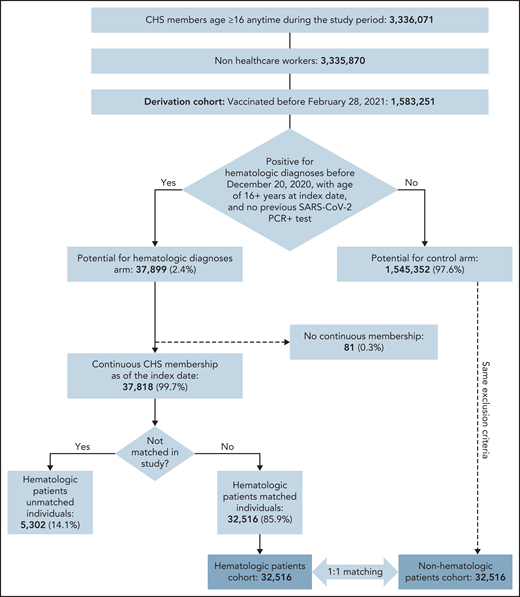
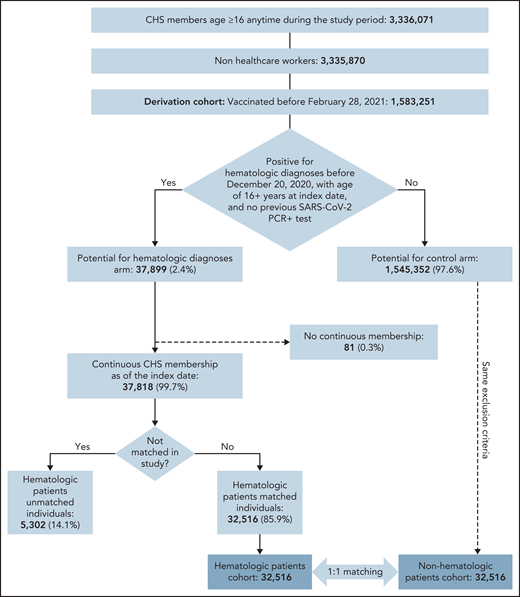
Of 1 583 251 CHS members who were vaccinated before 28 February 2021, 37 899 were eligible for the study and 32 516 were matched to unvaccinated controls (Figure 1). Patients were eligible to be included in the exposed group of patients with hematological neoplasms if they fulfilled 1 of the following criteria: (1) documented diagnosis of a hematological neoplasm (according to International Classification of Diseases, 9th edition, codes or Clalit diagnostic criteria) during the 6 months preceding data extraction, with or without chemotherapy or biologic therapy; (2) documented diagnosis of hematological neoplasm and a referral to an inpatient or outpatient clinic and/or daycare for administration of hematological treatment during the 6 months preceding data extraction; or (3) active chemotherapy and/or biologic therapy that is used exclusively for hematologic patients. (See supplemental Materials, available on the Blood Web site, for a list of diagnoses of hematological neoplasms and treatments.)
The patient population with hematological neoplasms was further divided into patients receiving therapy for hematological neoplasm or patients not receiving hematological treatment. Patients on therapy were defined as patients currently receiving or having recently received (≤6 months), biologic therapy or chemotherapy for hematological neoplasms. Patients without treatment were defined as patients who were followed but not treated, that is, either treated in the past (>6 months) or never requiring active treatment.
Study design
We used data collected between 20 December 2020, and 28 February 2021, prior to the emergence of the Delta variant. Vaccinated patients with hematological neoplasms were matched 1:1 to individuals without such disease who received the first vaccine dose at the same date (this date was defined as the index date for the matched pair). Exact matching was performed on age bins of 5 years, sex, town of residence, number of influenza vaccinations during the preceding 5 years (0, 1, 2+), pregnancy status, and bins of the total number of coexisting conditions (0-1, 2-3, 4+) that had been identified by the Centers for Disease Control and Prevention (CDC) as risk factors for severe COVID-19 as of 20 December 2020.16,17 Every case and control used in the study did not have prior documented severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–positive PCR result.
Five endpoints were evaluated during the follow-up period between days 7 and 43 after the second vaccine dose (days 28 and 64 from the first dose): (1) documented infection with SARS-CoV-2, confirmed by positive PCR test result; (2) symptomatic COVID-19 disease; (3) hospitalization due to COVID-19 infection; (4) severe COVID-19 disease (according to National Institutes of Health criteria18; and (5) death due to COVID-19.
The analysis was repeated separately in several subpopulations of patients with hematological neoplasms: patients on treatment of the hematological neoplasm; patients without treatment; patients with specific types of hematological neoplasms (eg, multiple myeloma, lymphoma, myeloid neoplasms); and patients receiving specific hematological medications (erythropoietin and rituximab).
Statistical analysis
After matching, survival curves were estimated using the Kaplan-Meier method.19 Effectiveness measures were evaluated at days 7 to 43 after the second vaccine dose. This process was done by only including pairs in which both matched persons were still at risk at the start of the period. 95% CI was estimated with the percentile bootstrap method using 3000 repetitions. Analyses were performed with the use of R software, version 4.0.
Ethics
This study was approved by the CHS Institutional Review Board.
Results
Overall, 32 156 individuals with a hematological neoplasm met the inclusion criteria and served as the exposed group in this study (Figure 1), which was matched to vaccinated controls without hematological neoplasm.
The median age of the studied patient population was 70 years (interquartile range 59, 79), with 48% being women (Table 1). The group included 5107 (15.9%) patients receiving therapy for hematological neoplasm; the remaining 27 049 (84.1%) were patients with hematological neoplasm but were not receiving treatment. More patients with hematological neoplasms than controls had a history of immunosuppressive treatment (14% vs 6.9%), liver disease (6.6% vs 3.9%), and chronic kidney disease (24% vs 20%). However, fewer patients with hematological neoplasms compared with the controls had diabetes mellitus type 2 (31% vs 34%), hypertension (53% vs 55%), history of being overweight (66% vs 71.4%), and smoking (14% vs 16%).
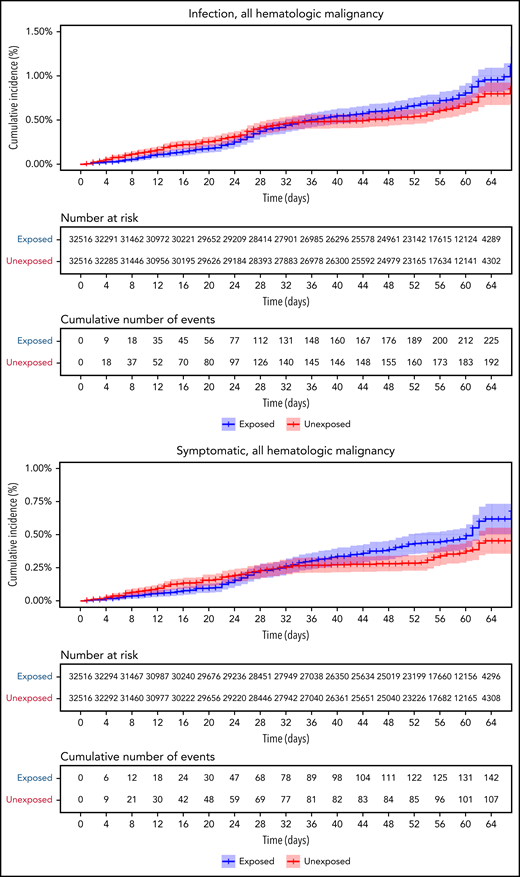
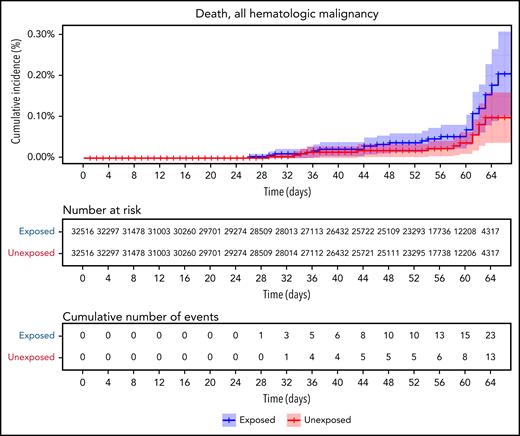
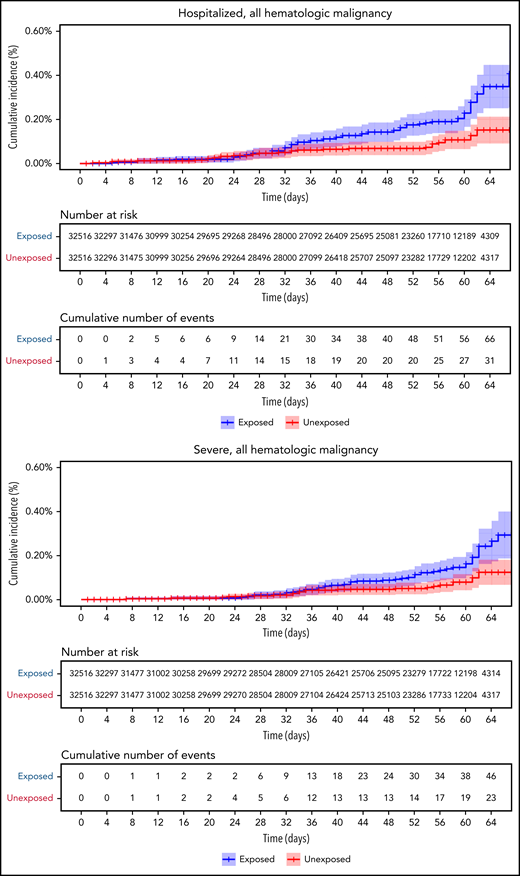
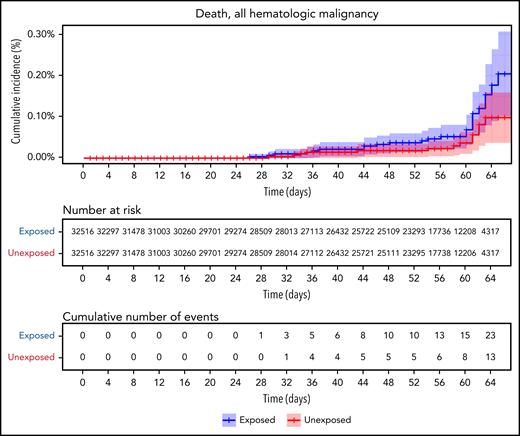
Vaccinated patients with hematological neoplasms were found to suffer from COVID-19 more than vaccinated matched controls (Table 2; Figure 2). Their risk ratio (RR) was higher for all 5 tested outcomes. They had a higher incidence of documented COVID-19 infections (RR 1.60, 95% CI 1.12-2.37); symptomatic disease (RR 1.72, 95% CI 1.05-2.85); COVID-19–related hospital admissions (RR 3.13, 95% CI 1.68-7.08); severe COVID-19 (RR 2.27, 95% CI 1.18-5.19); and COVID-19–related death (RR 1.66, 95% CI 0.72-4.47).
COVID-19 outcomes of all vaccinated patients with hematological neoplasms vs controls. Kaplan-Meier curves of outcomes: COVID-19 infection, symptoms, hospitalizations, severe disease, and death, respectively. The blue curve represents vaccinated patients; red curve represents vaccinated controls. The Kaplan-Meier curve covers the 64 days from the first vaccination dose, whereas the analysis and the data in the tables correspond to days 7 to 43 from the second vaccination dose (days 28-64 from the first dose). Note that in all outcomes, the patients with hematological neoplasms fared worse.
COVID-19 outcomes of all vaccinated patients with hematological neoplasms vs controls. Kaplan-Meier curves of outcomes: COVID-19 infection, symptoms, hospitalizations, severe disease, and death, respectively. The blue curve represents vaccinated patients; red curve represents vaccinated controls. The Kaplan-Meier curve covers the 64 days from the first vaccination dose, whereas the analysis and the data in the tables correspond to days 7 to 43 from the second vaccination dose (days 28-64 from the first dose). Note that in all outcomes, the patients with hematological neoplasms fared worse.
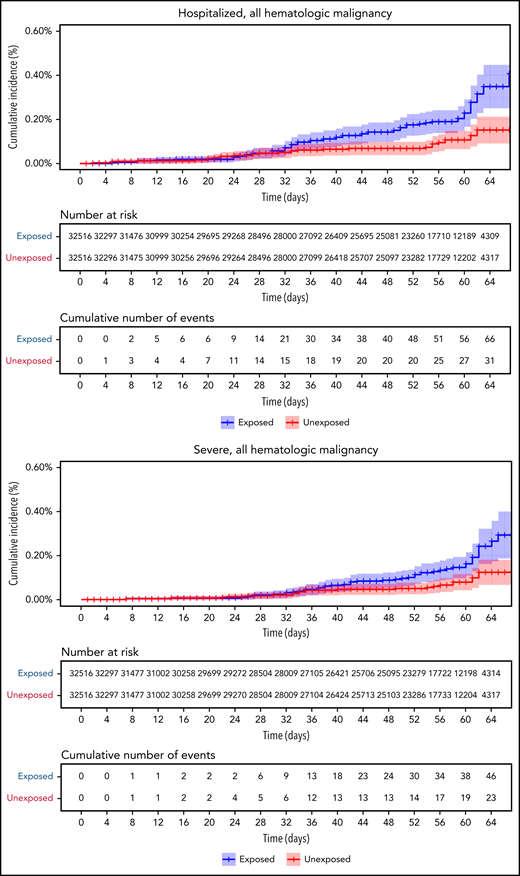
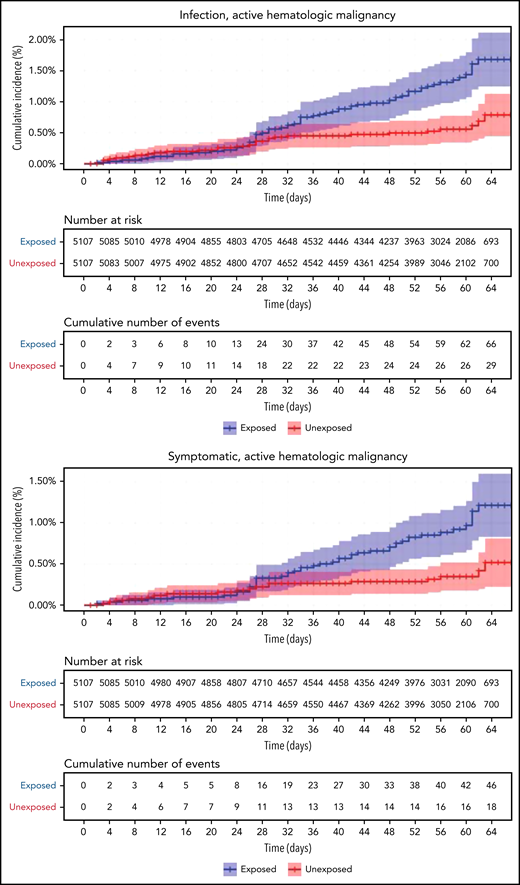
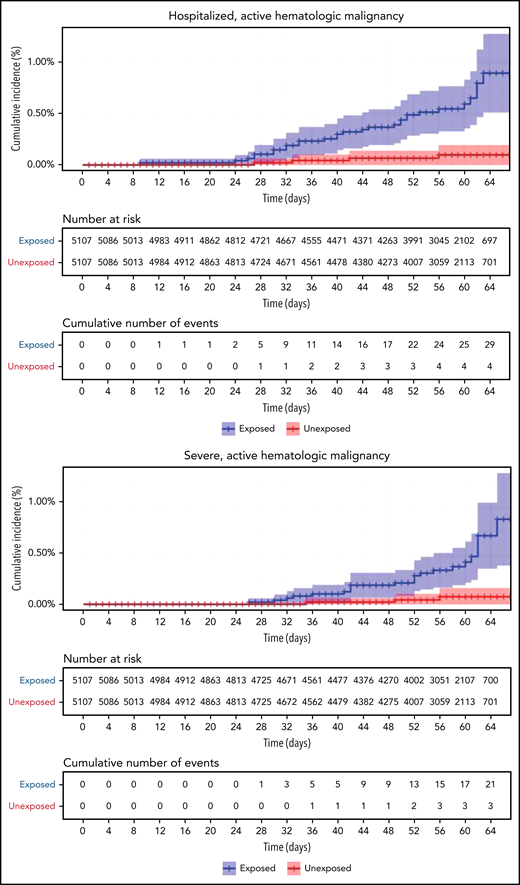
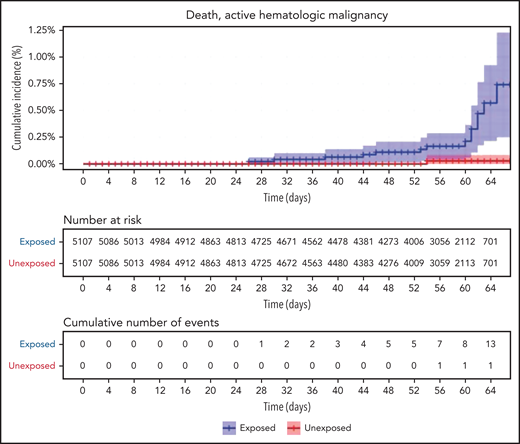
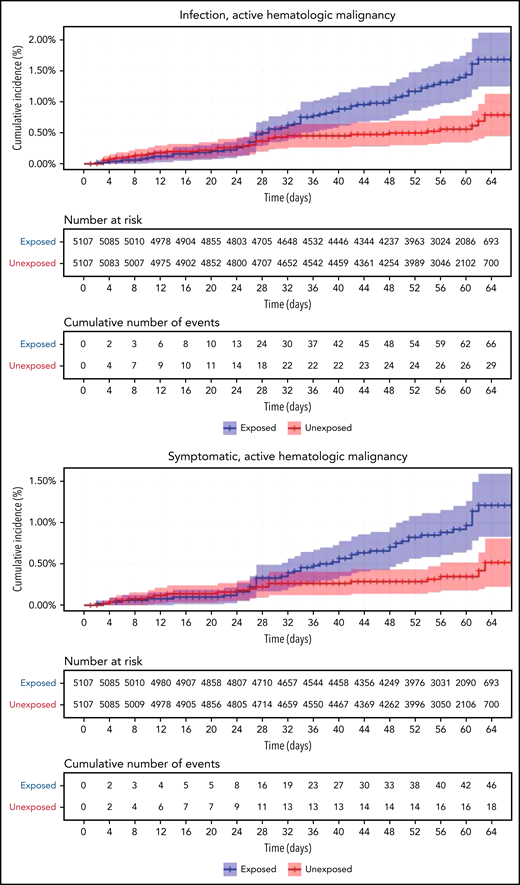
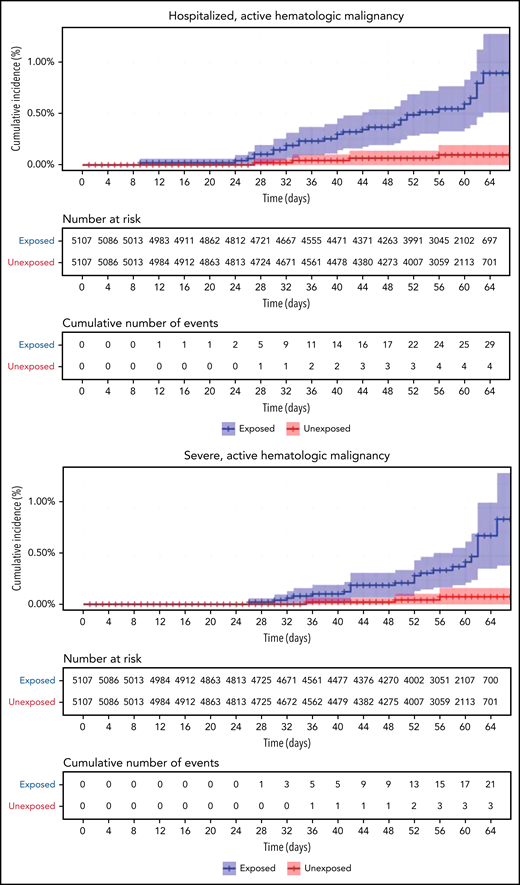
Restriction of the analysis to patients with actively treated hematological neoplasms increased RR (Table 2; Figure 3). RRs were 2.74 (1.31-8.99) for documented COVID-19 infection, 3.09 (1.20-20.81) for symptomatic disease, 10.81 (3.80-41.78) for hospital admissions, 8.97 (3.13-35.07) for severe disease, and 19.31 (3.95-30.72) for death. The risk was still increased in patients with hematological neoplasms without treatment although less so. Another analysis calculating the percentage of COVID-19 complications as percentage of infections supports this observation of increased risk in patients with hematological neoplasms and a trend toward higher risk as the complication becomes more serious (supplemental Tables 1 and 2).
COVID-19 outcomes of vaccinated patients receiving treatment of the hematological neoplasms vs controls. Kaplan-Meier curves of the same outcomes demonstrated in Figure 2, with blue and red curves representing vaccinated patients and vaccinated controls, respectively. The difference (RR) between the patient and control groups is even more prominent than that represented in Figure 2.
COVID-19 outcomes of vaccinated patients receiving treatment of the hematological neoplasms vs controls. Kaplan-Meier curves of the same outcomes demonstrated in Figure 2, with blue and red curves representing vaccinated patients and vaccinated controls, respectively. The difference (RR) between the patient and control groups is even more prominent than that represented in Figure 2.
Focusing on particular hematological neoplasms resulted in smaller groups with small numbers of events, but the trend was mostly similar. The vaccinated patient population with hematological neoplasms included 16 577 patients with lymphoma (Table 3). Compared with the vaccinated matched controls, these patients suffered more from COVID-19 infections (122 patients vs 77; RR 2.75, 95% CI 1.60-5.49); symptomatic disease (77 vs 39; RR 4.34, 95% CI 2.07-12.82); hospital admission (34 vs 5; RR 13.87, 95% CI 4.97-56.85); severe disease (25 vs 4; RR 12.06, 95% CI 4.03-48.47); and COVID-19–related death (13 vs 2; RR 15.13, 95% CI 3.68-46.29).
We analyzed data from 2855 patients with multiple myeloma (MM), of whom 1014 patients received antimyeloma treatment. Vaccinated patients with MM receiving antimyeloma treatment were more vulnerable to COVID-19 compared with vaccinated matched controls (Table 3) in terms of infection (16 patients vs 3; RR 4.65, 95% CI 1.57-17.73); symptomatic disease (10 vs 2; RR 5.29, 95% CI 1.46-15.61); hospital admission (6 vs 1; RR 6.43, 95% CI 1.00-10.95); and severe disease (6 vs 1; RR 6.67, 95% CI 0.98-11.43).
Other hematological neoplasms (acute leukemia, myeloproliferative neoplasms, and myelodysplastic syndromes) were analyzed as well. However, the number of patients, especially the number of events (individuals developing COVID-19 complications), were too small to allow for meaningful conclusions. Data are presented in supplemental Table 3.
We also analyzed hematological treatments, comparing the COVID-19 outcomes among vaccinated patients with hematological neoplasms on a specific medication to vaccinated matched controls who were not receiving the specific analyzed treatment. The number of COVID-19–affected patients treated with azacitidine, bortezomib, daratumumab, hydroxyurea, ibrutinib, lenalidomide, obinutuzumab, and bisphosphonates, as well as the number of events, was too small to allow for meaningful conclusions. However, vaccinated patients on erythropoietin or rituximab, despite the small numbers, showed a similar trend. More patients on these agents were affected by COVID-19 than vaccinated healthy controls (Table 4).
Discussion
We show that vaccinated patients with hematological neoplasms, compared with matched controls, had an increased rate of documented COVID-19 infections (RR 1.60), symptomatic disease (RR 1.72), hospital admissions (RR 3.13), severe disease (RR 2.27), and COVID-19–related death (RR 1.66). Patients receiving treatment of hematological neoplasms were at higher risk, with RR of 2.74, 3.09, 10.81, 8.97, and 19.31, respectively. Whether the reason for the higher relative risk in patients receiving treatment was the treatment, more advanced disease, association with impaired immunity, or more frequent hospital visits remains unclear.
The trend of increased risk was similar for patients with lymphoma and active myeloma. Vaccinated patients with hematological neoplasms treated with erythropoietin or rituximab were also shown to be at a relatively higher risk. It is likely that more advanced anemia and hematological neoplasms requiring erythropoietin treatment, usually myelodysplastic syndromes, are responsible for the increased risk of developing COVID-19 complications.
Our results regarding decreased vaccine effectiveness in patients with hematological neoplasms correlate with preliminary reports suggesting that these patients fail to produce high titers of anti–SARS-CoV-2 antibodies.7,14,20 Avivi et al.11 and Van Oekelen et al.8 reported low seropositive response in patients with multiple myeloma, compared with healthy controls or patients with smoldering disease. Observations in patients with lymphoma,12,15 chronic lymphocytic leukemia,10,13 and posttransplant or postcellular therapy21 produced similar results, as did observations of patients under treatment, especially anti-CD20 treatment, which showed that these patients are at higher risk.9,14 Impaired postvaccination T-cell immune response in immunocompromised patients has been reported as well.22
However, these preliminary reports demonstrating a reduced humoral immune or cellular response after mRNA vaccination among patients with hematological neoplasms included a relatively small number of patients, thus still requiring further large-scale confirmation. In addition, it is difficult to interpret anti–COVID-19 seroconversion to clinical efficacy, nor can we know the required titer of neutralizing antibodies to confer clinical immunity.7 Finally, the role of cellular immunity in COVID-19 infection is still unclear, with preliminary data suggesting abnormalities in these vulnerable patients.22,23 All this information points to a gap in correlating impaired immune responses to vaccination in patients with hematologic neoplasms with COVID-19 incidence and its complications.
The current study was performed in an attempt to fill this gap of knowledge regarding the practical effectiveness of the vaccines in this patient population. Such evidence might lead to applicable therapeutic strategies and a paradigm shift.
Our study has several limitations. A retrospective study based on electronic medical records may be associated with difficulties in identifying the right patient subpopulations as well as heterogeneity of the studied group (eg, all types of lymphoma). The number of individuals for some parameters, especially the number of events, may be too small to allow for meaningful comparisons. Also, no comparison was performed in this study with unvaccinated individuals. Having serology results could be beneficial when interpreting data. In addition, one must take into consideration that patients with hematological neoplasms are prone to developing various complications, not only COVID-19 related. Finally, many of the estimates are not precise, having wide confidence intervals.
Nevertheless, the findings teach us an important lesson and help in filling a missing component of the rapidly developing puzzle of COVID-19 vaccination. Patients with hematological neoplasms may not benefit from the vaccine as matched controls, especially if they receive treatment of the hematological neoplasm.
Such a study may help in paving the way toward improving the COVID-19 immunogenicity in these patients, and appropriate strategies should be explored. Administering a third dose of the vaccine has been suggested as a practical solution.7 In fact, 2 recent publications reported some success with a third vaccine given to patients with B-cell hematological neoplasms24 and after stem cell transplant.25 In Israel, a national program for providing boosters to individuals ≥30 years has been initiated, and recently (7 August 2021), the CDC recommended administering a third dose of the vaccine for immunocompromised patients.26
In summary, we estimated the effectiveness of the Pfizer BNT162b2 vaccine in patients with hematological neoplasms. Vaccinated patients, when compared with vaccinated controls, demonstrated reduced effectiveness for all studied outcomes. This effect was especially prominent for patients receiving treatment of hematological neoplasm. Strategies to enhance immunity in this patient population, including additional vaccine doses, should be explored.
Authorship
Contribution: M.M. and H.S.O. suggested the idea, designed the research project, analyzed the results, and wrote the draft of the manuscript; O.M., N.B., N.D., and R.B. helped to design the project, collected and extracted the data, analyzed the data, shared the data with the team, and completed writing the manuscript; A.L. participated in designing the project, contributed to the discussions, and participated in the writing process; and R.B. participated in designing the project, provided the infrastructure and approval for the project, participated in the discussions, and helped in the writing process.
Conflict-of-interest disclosure: O.M., N.B., N.D., and R.B. report institutional grants to the Clalit Research Institute from Pfizer outside the submitted work and unrelated to COVID-19, with no direct or indirect personal benefits. The remaining authors declare no competing financial interests.
Correspondence: Moshe Mittelman, Department of Hematology, Tel Aviv Sourasky Medical Center, 6 Weizmann St, Tel Aviv, 6423906 Israel; e-mail: moshemt@gmail.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.