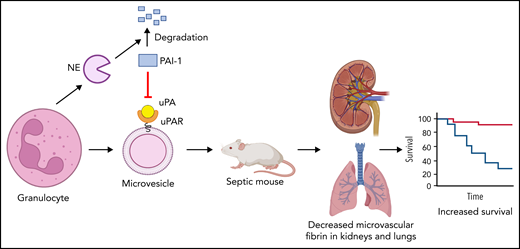
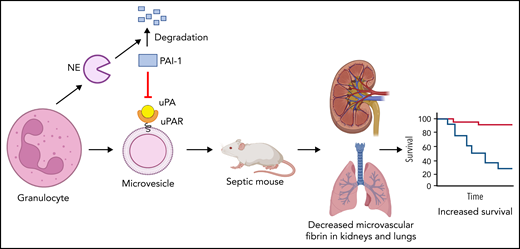
The study by Cointe et al1 in this issue of Blood shows that microvesicles (MVs) from human granulocytes express urokinase plasminogen activator receptor (uPAR) and when loaded with urokinase (uPA) lyse clots in vitro and reduce microthrombi in kidneys and lungs of septic mice and increase their survival. In addition, neutrophil elastase (NE) increased fibrinolysis by degrading plasminogen activator inhibitor 1 (PAI-1).
Extracellular vesicles are small membrane vesicles (0.1-1 μm), which includes MVs and exosomes, that are highly abundant in blood.2 The study of extracellular vesicles has exploded in recent years, with the therapeutic potential of extracellular vesicles being particularly exciting. Extracellular vesicles can be considered minicells that express cell-surface receptors from their parent cell and contribute to vascular homeostasis and cell-cell communication.2 The major source of MVs in blood is platelets, with these particles being originally referred to as platelet dust. It is now known that many cells release MVs into the blood, including endothelial cells, erythrocytes, granulocytes, and monocytes.2 Much of the early work on MVs focused on their procoagulant properties. For instance, the presence of tissue factor and negatively charged phospholipids, such as phosphatidylserine, make MVs highly procoagulant, and many studies have shown that they contribute to thrombosis.3 More recently, it was discovered that MVs derived from endothelial cells and leukocytes possess fibrinolytic activity.4 Interestingly, endothelial cell-derived MVs bound tissue plasminogen activator, whereas leukocyte-derived MVs expressed uPAR and bound uPA.4 MVs derived from neutrophils and monocytes had similar fibrinolytic activity but monocyte-derived MVs have much higher procoagulant activity because of the presence of tissue factor.1 An early study reported that human neutrophils express uPAR; therefore, MVs derived from these cells would be expected to also express uPAR.5
Septic shock is a systemic response to infection with a high mortality rate, and approved treatments are generally focused on supportive care and eradicating the source of the infection. Patients with sepsis have systemic inflammation, microvascular thrombosis, decreased fibrinolysis because of increased levels of PAI-1, and often have disseminated intravascular coagulation (DIC) and multiorgan failure.6 Unfortunately, despite considerable efforts targeting inflammation and coagulation has not improved outcomes in patients with sepsis. In terms of fibrinolytic capacity, 1 study found that patients with sepsis who developed DIC had higher levels of PAI-1 compared with those without DIC, and higher levels of PAI-1 were associated with decreased survival.7 Recently, Cointe and colleagues8 extended the analysis of fibrinolysis in patients with septic shock and found that lower levels of MV-plasmin generating capacity (MV-PGC) were associated with decreased survival. These studies suggest that reduced fibrinolytic capacity from either high PAI-1 or low MV-PGC increases the risk of microvascular thrombosis, DIC, organ failure, and death in patients with septic shock.
In this study, Cointe et al extended their work on the analysis of MV-PGC in patients with septic shock. Importantly, they found that granulocyte-MVs (Gran-MVs) from patients with high levels of MV-PGC had higher levels of both uPA and uPAR compared with patients with low MV-PGC, which suggested that granulocytes were the primary source of the MV-PGC. Gran-MVs from patients with septic shock lysed clots in vitro in a uPA- and uPAR-dependent manner. Addition of exogenous uPA increased the clot lysis activity of Gran-MVs. Taken together, these studies suggest that the balance between the coagulation system and the fibrinolytic system appears to play an important role in the outcome of patients with sepsis.
Next, the authors determined the effect of IV injection of MVs derived from human granulocytes from healthy individuals with or without exogenous uPA or a supernatant control on septic mice. Gran-MVs were injected daily for 4 days, and survival of the mice was evaluated at day 5. Levels of plasmin-α2 antiplasmin were increased in mice receiving Gran-MVs + uPA compared with the other 2 groups of mice at day 2, indicating increased plasmin generation. In addition, Gran-MVs + uPA prevented the increase in D-dimer at day 5 in mice, suggesting reduced fibrin generation. Importantly, injection of Gran-MVs + uPA reduced microvascular thrombi in the kidneys and lungs and increased survival of the septic mice compared with the other 2 groups (see figure). Injection of uPA without Gran-MVs did not protect the mice. There is long road between studies with septic mice and treatment of patients with sepsis. Nevertheless, the possibility of using Gran-MVs with PGC in the treatment of patients with sepsis, particularly those with low MV-PGC and/or high PAI-1, is exciting.
Human granulocytes increase fibrinolysis by releasing NE and MVs expressing uPAR. NE degrades PAI-1. Human granulocyte release MVs that express uPAR and bind uPA. Injection of granulocyte MVs and uPA into septic mice reduced microvascular thrombosis and increased survival. The figure was created using BioRender.com.
Human granulocytes increase fibrinolysis by releasing NE and MVs expressing uPAR. NE degrades PAI-1. Human granulocyte release MVs that express uPAR and bind uPA. Injection of granulocyte MVs and uPA into septic mice reduced microvascular thrombosis and increased survival. The figure was created using BioRender.com.
Finally, Cointe et al analyzed plasma factors from patients with sepsis that affected fibrinolysis. Plasma from patients with sepsis was analyzed using a multiplex array for 23 molecules that are known to modulate the uPA/uPAR system. Levels of NE correlated with Gran-MV-PGC. NE has also been shown to degrade PAI-1.9 Indeed, addition of NE increases the fibrinolytic activity of Gran-MVs because of degradation of PAI-1 (see figure). Therefore, neutrophils increase fibrinolysis in 2 ways: they release uPAR-expressing MVs that bind uPA and increase plasmin generation, and also via release of NE that degrades PAI-1.
The study has some limitations. Although the data supporting the notion that Gran-MVs improve survival in septic mice by rebalancing the coagulation and fibrinolytic systems is compelling, one cannot exclude the possibility that Gran-MVs have other beneficial activities that increase survival of the septic mice. For instance, neutrophil MVs contain annexin 1, which has anti-inflammatory activity. For the mouse experiments, MVs were derived from unstimulated human granulocytes rather than from murine neutrophils. MVs released from human granulocytes cultured under different conditions may have different fibrinolytic activities. One study prepared 2 types of MVs from stimulated human neutrophils and found that only 1 type of MV protected septic mice.10 Another study found that neutrophil-derived MVs isolated from the peritoneal cavity of septic mice reduced survival of septic mice.11 Clearly, further work is needed but the current study suggests that MVs derived from human granulocytes may be useful in treating disorders associated with microvascular thrombosis, such as sepsis and COVID-19.
Conflict-of-interest disclosure: The authors declare no competing financial interests.