Abstract
Superoxide production by the phagocyte reduced NAD phosphate (NADPH) oxidase is essential for innate immunity as shown in chronic granulomatous disease (CGD), an immunodeficiency disease resulting from mutations in 1 of its genes. The NADPH oxidase is composed of 2 membrane proteins (gp91phox/NOX2 and p22phox) and 4 cytosolic proteins (p47phox, p67phox, p40phox, and Rac1/2). The phosphorylation of p47phox is required for NADPH oxidase activation in cells. As p47phox and p67phox can form a tight complex in cells, we hypothesized that p67phox could regulate p47phox phosphorylation. To investigate this hypothesis, we used phospho-specific antibodies against 5 major p47phox-phosphorylated sites (Ser304, Ser315, Ser320, Ser328, and Ser345) and neutrophils from healthy donors and from p67phox−/− CGD patients. Results showed that formyl-methionyl-leucyl-phenylalanine and phorbol myristate acetate induced a time- and a concentration-dependent phosphorylation of p47phox on Ser304, Ser315, Ser320, and Ser328 in healthy human neutrophils. Interestingly, in neutrophils and Epstein-Barr virus-transformed B lymphocytes from p67phox−/− CGD patients, phosphorylation of p47phox on serine residues was dramatically reduced. In COSphox cells, the presence of p67phox led to increased phosphorylation of p47phox. In vitro studies showed that recombinant p47phox was phosphorylated on Ser304, Ser315, Ser320, and Ser328 by different PKC isoforms and the addition of recombinant p67phox alone or in combination with p40phox potentiated this process. Thus, p67phox and p40phox are required for optimal p47phox phosphorylation on Ser304, Ser315, Ser320, and Ser328 in intact cells. Therefore, p67phox and p40phox are novel regulators of p47phox-phosphorylation.
Key Points
Phosphorylation of the NADPH oxidase component p47phox is decreased in neutrophils from p67phox-deficient patients with CGD.
p67phox and p40phox are novel regulators of p47phox-phosphorylation.
Introduction
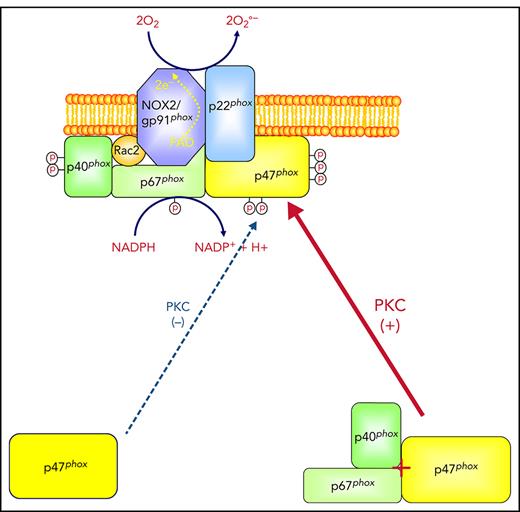
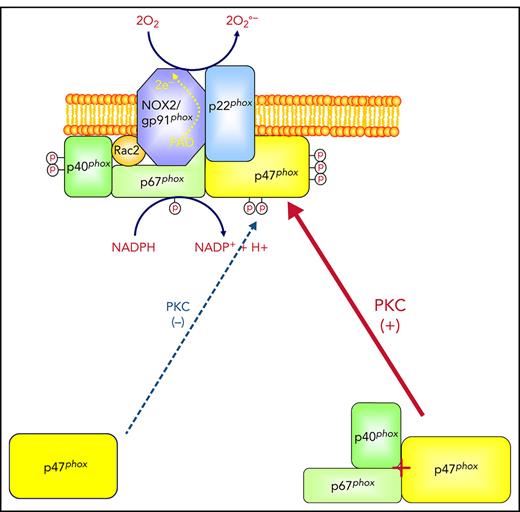
Polymorphonuclear neutrophils are the most numerous circulating leukocytes and play an important role in innate immunity and inflammation.1,2 During phagocytosis, neutrophils are activated to produce high quantities of superoxide anion that generates other reactive oxygen species (ROS) such as hydrogen peroxide, hydroxyl radical, and hypochlorous acids.3,4 The enzyme responsible for superoxide anion production by neutrophils is called the phagocyte reduced NAD phosphate (NADPH) oxidase.5-7 This enzyme complex is composed of 2 membrane-bound proteins, p22phox and gp91phox/NOX2, which form the cytochrome b558, and 4 cytosolic proteins, p47phox, p67phox, p40phox, and Rac2.5-7 In resting cells, these proteins are distributed between the cytosol and membranes of granules and the plasma membrane. Upon activation, p47phox, p67phox, p40phox, p22phox, and gp91phox are phosphorylated, Rac2 is activated, and the system assembles into an active complex.8-11
ROS production is required for host defense, as illustrated by the genetic immunodeficiency disease called chronic granulomatous disease (CGD), in which phagocytes do not produce ROS and affected patients have more fatal infections than healthy individuals.12-16 CGD is a rare disease found in 1 of 200 000 to 250 000 individuals. It is caused by a gene mutation in 1 of the NADPH oxidase components, the most frequent CGD form being caused by mutations in the gp91phox/CYBB gene (65%), followed by mutation in the p47phox/NCF1 gene (20%), the p22phox/CYBA gene (<5%), the p67phox/NCF2 gene (<5%), the p40phox/NCF4, and Rac2/NCF3 genes (both forms <5%).12-16
In addition to host defense, ROS production can participate in tissue injury and excessive inflammation if not well controlled.10,17-19 Thus, activation of the phagocyte NADPH oxidase must be tightly regulated to ensure that ROS are produced only when and where required. One mechanism by which the NADPH oxidase is regulated is the phosphorylation of its organizer subunit, p47phox.10,11 Stimulation of neutrophils by the chemotactic peptide formyl-methionyl-leucyl-phenylalanine (fMLF) or the protein kinase C (PKC) activator phorbol myristate acetate (PMA) induces NADPH oxidase activation via p47phox phosphorylation.20-23 This phosphorylation occurs on several serine residues localized in the C-terminal p47phox sequence (Ser 303-379), and phosphorylation of specific serines is required for NADPH oxidase activation, as shown by site-directed mutagenesis and transfection of Epstein-Barr virus (EBV)-transformed B lymphocytes.22,23 In contrast to activating agents, priming agents such as the proinflammatory cytokines, tumor necrosis factor-α (TNF-α) and granulocyte-macrophage colony-stimulating factor, and the Toll-like receptor agonist, lipopolysaccharide, do not stimulate ROS production and only induce partial p47phox phosphorylation on Ser345.24-27 Phosphorylated Ser345 has been shown to be a docking site for the proline isomerase Pin1, which induces p47phox conformational changes and facilitates p47phox phosphorylation by various protein kinases.27-29
In resting cells, p47phox can be found in a free form or in a complex with p67phox and p40phox.30-32 The interaction between p47phox and p67phox is mediated via binding of SH3 domains to a polyproline region.33-35 We sought to investigate the role of p67phox in p47phox phosphorylation using site-specific anti-phospho-p47phox antibodies and neutrophils from p67phox−/− patients with CGD. We observed that fMLF and PMA induced phosphorylation of Ser304, Ser315, Ser320, and Ser328 of the p47phox subunit in human neutrophils but phosphorylation of all sites was dramatically impaired in neutrophils from p67phox-deficient patients with CGD.
Methods
Materials
Phosphate-buffered saline, Hanks balanced salt solution (HBSS), PMA, fMLF, diisopropylfluorophosphate, reduced glutathione, proteases, and kinases inhibitors were from Sigma Chemical (St. Louis, MO). Dextran and Ficoll were from GE-Healthcare. Reagents for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting were purchased from Bio-Rad (Richmond, CA). Polyclonal phospho-specific antibodies were produced by our group as previously described.23,28 The anti-p47phox, anti-p67phox, and anti-gp91phox were from Santa Cruz Biotechnologies. Peroxidase-labeled goat anti-rabbit antibody was purchased from Jackson Laboratories. Blotto dry milk and Luminol reagents for protein detection were purchased from Santa Cruz Biotechnologies.
Ethics statement
Neutrophils were isolated from the venous blood of healthy volunteers and from 2 unrelated p67phox-deficient patients with CGD after written informed consent was obtained. The study was approved by the institutional review boards and ethics committee of INSERM (EFS convention number 2018010827). All procedures were conducted in accordance with the 1975 Declaration of Helsinki, as revised in 2013. Data collection and analyses were performed anonymously.
Preparation of human neutrophils
Human neutrophils from venous blood were obtained by Dextran sedimentation and Ficoll centrifugation.36,37 Neutrophils were 96% pure and 99% viable. The cells (5 × 107/mL) were suspended in phosphate-buffered saline and treated with the serine protease inhibitor diisopropylfluorophosphate (2.7 mM) for 20 minutes at 4°C, then washed and resuspended in HBSS buffer containing 1.2 mM Ca++, 1.2 mM Mg++, and 5 mM d-glucose.
Neutrophil stimulation and lysis
For the phosphorylation experiments, neutrophils resuspended at 15 × 106 cells/0.4 mL of HBSS were incubated for 10 minutes at 37°C before stimulation with PMA or fMLF for the indicated times and concentrations. The reaction was stopped by adding 5× modified Laemmli sample buffer37,38 containing 5 mmol/L Na3VO4, 2.5 mmol/L p-NPP, 10 mmol/L NaF, 5 mmol/L EDTA, 5 mmol/L EGTA, 20 µg/L leupeptin, 20 µg/L pepstatin, and 20 µg/L aprotinin. Samples were then incubated for 2 minutes in boiling water (100°C) and stored at −80°C until use.
EBV-transformed B-lymphocyte preparation and stimulation
Peripheral blood from healthy donors and p67phox-deficient patients with CGD was subjected to fractionation by Ficoll centrifugation. Mononuclear cells were collected, washed twice, and cultured in RPMI1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin in the presence of supernatants from the EBV-producing cell line, B95-8.23 Three weeks later, EBV-transformed B lymphocytes (EBV-L) had proliferated and represented more than 90% of the cultured cells as determined by flow cytometry. For the phosphorylation experiments, EBV-transformed lymphocytes were stimulated with PMA and prepared as described previously for neutrophils.
ROS production assay
ROS production was measured by the luminol and horse radish peroxidase (HRP)-enhanced chemiluminescence method.39 Neutrophils (0.5 × 106 cells) or EBV-L (2 × 106 cells) were resuspended in 500 μL HBSS containing 10 μM luminol and 10 U HRP, preheated at 37°C in the thermostated chamber of the luminometer (Biolumat LB937; Berthold) for 10 minutes, and then stimulated with different concentrations of fMLF or PMA and chemiluminescence was recorded. In the presence of HRP, chemiluminescence was completely inhibited by SOD as we previously showed,39 indicating that this method detects superoxide anion production.
COSphox cells
The COSphox stable cell lines, which had been transfected with the NADPH oxidase components (gp91phox, p22phox, p47phox, and p67phox) or without p67phox, were maintained at 37°C with 5% CO2 in Dulbecco's modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 IU/mL penicillin, 50 μg/mL streptomycin, and other antibiotics for selection as detailed previously.40 For small interfering RNA (siRNA) knockdown, COSphox cell line expressing gp91phox, p22phox, p47phox, and p67phox were transiently transfected with either p67phox siRNAs (sc-36163) or scramble siRNA control (sc-37007), using the siRNA transfection reagent and protocol recommended by the manufacturer. Forty-eight hours after transfection, the effect of siRNA-mediated knockdown was determined using western blotting with anti-p67phox antibody. Two million cells were resuspended in HBSS, preheated, and then treated with PMA (200 ng/mL) for 30 minutes at 37°C, lysed, and proteins denatured.
Expression of recombinant p47phox, p67phox, and p40phox
For recombinant protein expression, the pGEX-6P1 plasmids containing complementary DNA (cDNA) encoding glutathione S-transferase (GST)-p47phox, GST-p67phox or pET-p40phox plasmid were transformed into BL21 Escherichia coli, and the cells were cultured overnight in 100 mL of bacterial culture medium containing ampicillin (100 μg/mL) at 37°C. The overnight culture was then diluted 10-fold and allowed to grow until the optical density reached 0.8 at 600 nm. Expression of the proteins was induced by incubation with isopropyl-β-d-thiogalactoside (20 μM) for 3 hours at 37°C. Cells were pelleted by centrifugation at 2500g for 25 minutes, lysed by sonication in lysis buffer (50 mM NaCl; 5 mM MgCl2; 50 mM Tris-HCl, pH 7.5; 1 mM DTT; 1% Triton X-100; and a mixture of protease inhibitors), and centrifuged at 10 000g for 20 minutes at 4°C. The soluble GST-p47phox, GST-p67phox, and His-Tagged-p40phox were affinity-purified by incubation of the supernatant with glutathione-Sepharose 4B beads or nickel affinity chromatography, respectively, as previously described.36
In vitro phosphorylation of p47phox by PKC isoforms
Phosphorylation by PKC was performed as previously described.36,41 Briefly, the reaction mixture contained 1 μg p47phox recombinant protein in the absence or presence of p67phox and p40phox, and 25 ng purified PKC in 40 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 10 mM MgCl2, 1 mM DTT, 5 μg/mL diacylglycerol, 150 μg/mL phosphatidylserine, 3 mM CaCl2, and 50 μM adenosine triphosphate (ATP) in a total volume of 50 μL. The reactions were incubated for 30 minutes at 30°C and terminated at the indicated times by addition of 10 μL of 5× Laemmli sample buffer and denaturation at 100°C.
Electrophoresis and blotting
Heat-denatured cell samples were sonicated twice for 5 seconds to break down DNA and centrifuged at 2000g for 8 minutes. The samples (1 million cell-equivalent) were subjected to 10% SDS-PAGE using standard techniques.37,38,42 The separated proteins were transferred to nitrocellulose membranes, which were blocked with 5% milk in Tris-buffered saline containing Tween 20 for 1 hour. After blocking, the membranes were probed with the anti-phospho-Ser304-p47phox (1:2000), anti-phospho-Ser315-p47phox (1:2000), anti-phospho-Ser320-p47phox (1:2000), anti-phospho-Ser328-p47phox (1:2000), anti-phospho-Ser345-p47phox (1:10 000), and anti-p47phox (1:5000) antibodies for 1 hour at room temperature or overnight at 4°C. Membranes were then washed and incubated with HRP-labeled goat anti-rabbit antibody (1:10 000) for 1 hour at room temperature. The anti-gp91phox and anti-p67phox antibodies were used at 1:5000 and 1:2000, respectively. The protein bands were revealed using enhanced chemiluminescence reagents. Quantification of protein bands was performed by densitometry using Image J software analysis (Wayne Rasband, National Institutes of Health). Phosphorylated p47phox was quantified after correction for the total amount of p47phox protein.
Statistical analysis
All results are expressed as means ± standard deviation. Data were analyzed with GraphPad Prism 7 software (GraphPad Software, San Diego, CA). Differences between groups were analyzed by 1-way analysis of variance test with Tukey's multiple comparison posttest. *P < .05, **P < .01, and ***P < .001 values were considered as significant.
Results
fMLF induced a rapid and transient phosphorylation of p47phox on Ser304, 315, 320, and 328 in human neutrophils
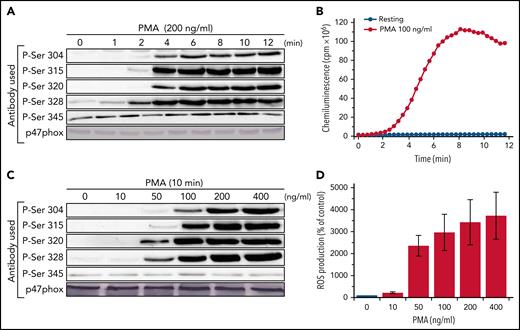
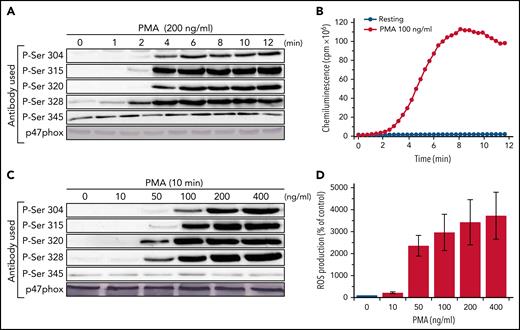
To investigate the possible role of p67phox in the phosphorylation of p47phox, we used neutrophils from very rare patients with CGD who are deficient for p67phox (<1 case per 5 000 000 individuals), and very sensitive site-specific anti-phospho-p47phox antibodies that we developed in our laboratory.23,28 We first determined the optimal kinetics and concentrations of 2 different agonists, the bacterial peptide fMLF and the PKC activator PMA using neutrophils from healthy donors. Neutrophils were denatured in a 5× modified Laemmli sample buffer, sonicated and centrifuged at low speed, and the supernatants were used for SDS-PAGE and western blotting as previously described.37 Under those conditions, p47phox was fully solubilized and only detected in the supernatant (supplemental Figure 1 on the Blood Web site). Results show that fMLF (at 1 µM) induced the phosphorylation of p47phox on Ser304, 315, 320, and 328 very rapidly because it was detected as soon as 5 seconds, the shortest time at which we could stimulate and stop the reaction (Figure 1A). Phosphorylated p47phox from different experiments was quantified and corrected for the total amount of p47phox protein by densitometry as described in the Methods. The mean of these experiments (supplemental Figure 2) shows that fMLF-induced p47phox phosphorylation on Ser304, 315, 320, and 328 reached a peak at 10 seconds, followed by a decline, likely because of dephosphorylation as the total amount of p47phox did not change over time. Ser345, a site known to be phosphorylated in TNF-α-, granulocyte-macrophage colony-stimulating factor-, or lipopolysaccharide-treated neutrophils,26,27 was already phosphorylated in nonstimulated neutrophils, and fMLF barely increased its phosphorylation. The 4 phosphorylated serines were almost completely dephosphorylated after a 90-second stimulation. Interestingly, the kinetics of fMLF-induced p47phox-phosphorylation precede those of the NADPH oxidase activation (Figure 1B). fMLF-induced phosphorylation of Ser304, 315, 320, and 328 was concentration-dependent (Figure 1C; supplemental Figure 3), starting between 10−9 and 10−8 M, and reaching its maximal effect at 10−6 M, which is the optimal fMLF concentration to induce NADPH oxidase activation (Figure 1D).
Kinetics and concentration-dependent effect of fMLF on p47phox phosphorylation and ROS production in human neutrophils. (A) Human neutrophils (15 × 106/400 µL) were incubated with fMLF (1 µM) for different times and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328, and anti-phospho-Ser-345 antibodies and anti-p47phox antibody. (B) Neutrophils (0.5 × 106/500 µL) were incubated in HBSS for 5 minutes in the presence of 10 µM luminol at 37°C and stimulated with fMLF (1 µM); ROS production was measured by luminol-amplified chemiluminescence over time. (C) Human neutrophils (15 × 106/400 µL) were incubated with different concentrations of fMLF for 10 seconds and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using the same antibodies as in panel A. (D) ROS production of neutrophils (0.5 × 106/500 µL HBSS) was measured as in panel B. Experiments were repeated 3 times (n = 3, mean ± standard error of the mean [SEM]).
Kinetics and concentration-dependent effect of fMLF on p47phox phosphorylation and ROS production in human neutrophils. (A) Human neutrophils (15 × 106/400 µL) were incubated with fMLF (1 µM) for different times and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328, and anti-phospho-Ser-345 antibodies and anti-p47phox antibody. (B) Neutrophils (0.5 × 106/500 µL) were incubated in HBSS for 5 minutes in the presence of 10 µM luminol at 37°C and stimulated with fMLF (1 µM); ROS production was measured by luminol-amplified chemiluminescence over time. (C) Human neutrophils (15 × 106/400 µL) were incubated with different concentrations of fMLF for 10 seconds and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using the same antibodies as in panel A. (D) ROS production of neutrophils (0.5 × 106/500 µL HBSS) was measured as in panel B. Experiments were repeated 3 times (n = 3, mean ± standard error of the mean [SEM]).
PMA induced a less rapid but more sustained phosphorylation of p47phox on Ser304, 315, 320, and 328 than fMLF in human neutrophils
PMA is a known strong activator of the NADPH oxidase and an inducer of p47phox phosphorylation in human neutrophils. PMA-induced neutrophil activation occurs by direct activation of PKC.43 To evaluate the PMA optimal concentration and time course, we stimulated healthy neutrophils between 1 and 12 minutes at concentrations ranging from 10 to 200 ng/mL. We show that PMA (at 200 ng/mL) induced the time-dependent phosphorylation of p47phox on Ser304, 315, 320, and 328 (Figure 2A). PMA induced only a moderate increase in the phosphorylation of Ser345. Phosphorylated p47phox from different experiments was quantified by densitometry and corrected for total p47phox amount as described in the "Methods." The mean of these experiments (supplemental Figure 4) shows that PMA-induced phosphorylation occurs after a 2-minutes lag time, then increased and plateaued over time. In contrast to what we observed with fMLF, dephosphorylation was not observed within the 12-minute time frame tested. Interestingly, the kinetics of PMA-induced p47phox-phosphorylation parallel the kinetics of NADPH oxidase activation (Figure 2B). PMA-induced phosphorylation of Ser304, 315, 320, and 328 was concentration dependent (Figure 2C; supplemental Figure 5), starting at 10 to 50 ng/mL and reaching its maximal effect at 100 ng/mL and higher, which are the optimal concentrations to induce NADPH oxidase activation (Figure 2D).
Kinetics and concentration-dependent effect of PMA on p47phox phosphorylation and ROS production in human neutrophils. (A) Human neutrophils (15 × 106/400 µL) were incubated with PMA (200 ng/mL) for different times and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328, and anti-phospho-Ser-345 antibodies and anti-p47phox antibody. (B) Neutrophils (0.5 × 106/500 µL) were incubated in HBSS for 5 minutes in the presence of 10 µM luminol at 37°C then stimulated with PMA (100 ng/mL); ROS production was measured by luminol-amplified chemiluminescence over time. (C) Human neutrophils (15 × 106/400 µL) were incubated with different concentrations of PMA for 10 minutes and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using the same antibodies as in panel A. (D) ROS production of neutrophils (0.5 × 106/500 µL in HBSS) was measured as in panel B. Experiments were repeated 3 times (n = 3, mean ± SEM).
Kinetics and concentration-dependent effect of PMA on p47phox phosphorylation and ROS production in human neutrophils. (A) Human neutrophils (15 × 106/400 µL) were incubated with PMA (200 ng/mL) for different times and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328, and anti-phospho-Ser-345 antibodies and anti-p47phox antibody. (B) Neutrophils (0.5 × 106/500 µL) were incubated in HBSS for 5 minutes in the presence of 10 µM luminol at 37°C then stimulated with PMA (100 ng/mL); ROS production was measured by luminol-amplified chemiluminescence over time. (C) Human neutrophils (15 × 106/400 µL) were incubated with different concentrations of PMA for 10 minutes and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using the same antibodies as in panel A. (D) ROS production of neutrophils (0.5 × 106/500 µL in HBSS) was measured as in panel B. Experiments were repeated 3 times (n = 3, mean ± SEM).
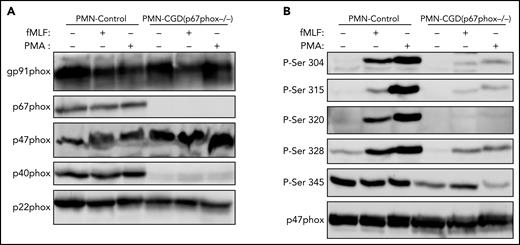
Phosphorylation of p47phox on Ser304, 315, 320, 328, and 345 in neutrophils and EBV-transformed lymphocytes from p67phox-deficient patients with CGD is dramatically reduced
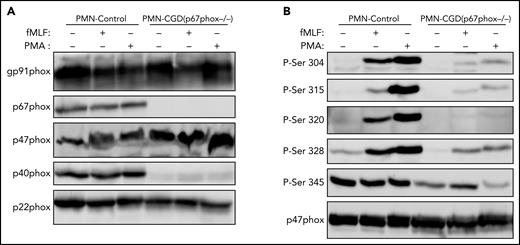
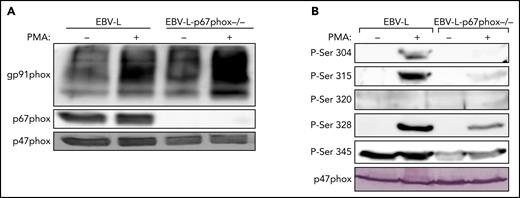
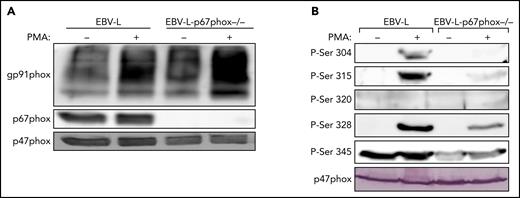
To investigate the possible role of p67phox in the phosphorylation of p47phox, we isolated neutrophils from a unique cohort of 2 patients with CGD deficient for p67phox. The 2 patients are not related and they have the same p67phox/NCF2 gene mutation [c.364 + 1G>A],12 which results in the absence of the p67phox protein (Figure 3A). We first confirmed that neutrophils from these patients did not produce ROS under resting conditions and after stimulation with PMA and fMLF (supplemental Figure 6). Furthermore, western blot analysis confirmed that the p67phox protein was absent (Figure 3A). In addition, the p40phox subunit was barely expressed (12.5%), whereas g91phox, p47phox, and p22phox were all normally expressed. Interestingly, when we investigated p47phox phosphorylation in resting and fMLF- and PMA-stimulated neutrophils, we observed that the phosphorylation of p47phox on Ser304, Ser315, Ser320, and Ser328 was dramatically reduced in neutrophils from p67phox−/− patients with CGD compared with controls (Figure 3B; supplemental Figure 7). These results were similar in neutrophils from both patients with CGD. Given the rarity of p67phox deficiency,12-16 we used EBV-L to confirm these results. Western blot analysis of EBV-L showed that the p67phox protein was completely absent in p67phox−/−-patient-derived EBV-L compared with control, whereas gp91phox and p47phox were normally expressed (Figure 4A). As observed in neutrophils from p67phox−/−patients with CGD, PMA-induced phosphorylation of p47phox on Ser304, Ser315, and Ser328 in EBV-L was dramatically reduced (Figure 4B). Interestingly, the phosphorylation of Ser345 in the absence or presence of PMA was also reduced in p67phox−/−-patient-derived EBV-L. Unexpectedly, Ser320 was not phosphorylated in PMA-stimulated EBV-L. Altogether, these data suggest that p67phox is required for optimal phosphorylation of p47phox in neutrophils and EBV-L.
Phosphorylation of p47phox in neutrophils from p67phox-deficient patients and from healthy donors. (A) Neutrophils from healthy donors (PMN-Control) and p67phox-deficient patients (PMN-CGD(p67−/−)) (15 × 106/400 µL) were incubated with fMLF (1 µM) or PMA (200 ng/mL) for 10 seconds and 10 minutes, respectively, and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using anti-gp91phox, anti-p67phox, anti-p47phox, anti-p40phox, and anti-p22phox antibodies. (B) Neutrophils from healthy donors (PMN-Control) and p67phox-deficient patients (PMN-CGD(p67−/−)) (15 × 106/400 µL) were incubated with fMLF (1 µM) or PMA (200 ng/mL) for 10 seconds and 10 minutes, respectively, and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328, and anti-phospho-Ser-345 antibodies and anti-p47phox antibody. Experiments were repeated twice in duplicates.
Phosphorylation of p47phox in neutrophils from p67phox-deficient patients and from healthy donors. (A) Neutrophils from healthy donors (PMN-Control) and p67phox-deficient patients (PMN-CGD(p67−/−)) (15 × 106/400 µL) were incubated with fMLF (1 µM) or PMA (200 ng/mL) for 10 seconds and 10 minutes, respectively, and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using anti-gp91phox, anti-p67phox, anti-p47phox, anti-p40phox, and anti-p22phox antibodies. (B) Neutrophils from healthy donors (PMN-Control) and p67phox-deficient patients (PMN-CGD(p67−/−)) (15 × 106/400 µL) were incubated with fMLF (1 µM) or PMA (200 ng/mL) for 10 seconds and 10 minutes, respectively, and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328, and anti-phospho-Ser-345 antibodies and anti-p47phox antibody. Experiments were repeated twice in duplicates.
Phosphorylation of p47phox in EBV-transformed B lymphocytes from p67phox-deficient patients and from healthy donors. (A) EBV-transformed B lymphocytes from healthy donors (EBV-L) and p67phox-deficient patients (EBV-l-p67phox−/−) (15 × 106/400 µL) were stimulated with PMA (200 ng/mL) for 10 minutes and the reaction was stopped by denaturation with 100 µL Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using anti-gp91phox, anti-p67phox, and anti-p47phox antibodies. (B) EBV-transformed B lymphocytes from healthy donors (EBV-L) and p67phox-deficient patients (EBV-l-p67phox−/−) (15 × 106/400 µL) were stimulated with PMA (200 ng/mL) for 10 minutes and the reaction was stopped by denaturation with 100 µL Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328, and anti-phospho-Ser-345 antibodies and anti-p47phox antibody. Experiments were repeated 3 times.
Phosphorylation of p47phox in EBV-transformed B lymphocytes from p67phox-deficient patients and from healthy donors. (A) EBV-transformed B lymphocytes from healthy donors (EBV-L) and p67phox-deficient patients (EBV-l-p67phox−/−) (15 × 106/400 µL) were stimulated with PMA (200 ng/mL) for 10 minutes and the reaction was stopped by denaturation with 100 µL Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using anti-gp91phox, anti-p67phox, and anti-p47phox antibodies. (B) EBV-transformed B lymphocytes from healthy donors (EBV-L) and p67phox-deficient patients (EBV-l-p67phox−/−) (15 × 106/400 µL) were stimulated with PMA (200 ng/mL) for 10 minutes and the reaction was stopped by denaturation with 100 µL Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328, and anti-phospho-Ser-345 antibodies and anti-p47phox antibody. Experiments were repeated 3 times.
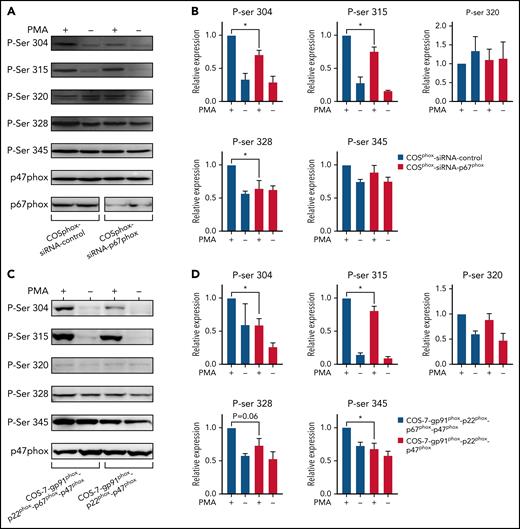
Knockdown of p67phox expression or its absence in COSphox cells impaired phosphorylation of p47phox
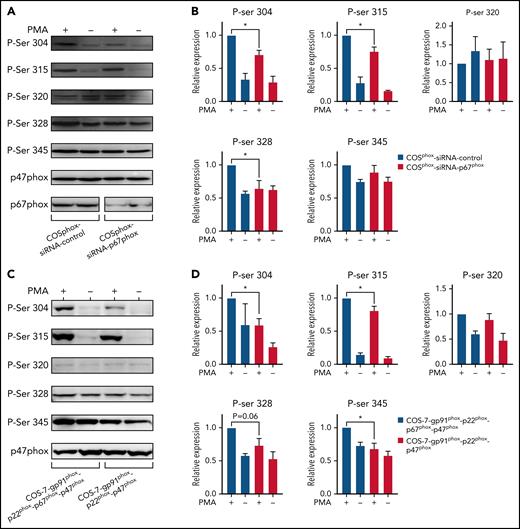
To confirm the results obtained with neutrophils and EBV-L, we used an siRNA approach to inhibit p67phox expression in COSphox cells. Results show that the siRNA against p67phox dramatically decreased the expression of the protein in these cells (Figure 5A), which resulted in the significant decrease of PMA-stimulated p47phox phosphorylation on Ser304, 315, and 328 (Figure 5A-B). Interestingly, Ser320 and 345 showed only a marginal increase in p47phox phosphorylation, and absence of p67phox had no effect on their phosphorylation. To confirm the siRNA data, we used COSphox cells with or without p67phox expression. As expected, the phosphorylation of p47phox was lower in cells expressing only gp91phox, p22phox, and p47phox compared with cells also expressing p67phox (Figure 5C-D).
p67phox knockdown reduces the phosphorylation of p47phox and its expression increases this process in COSphox cell lines. (A) COSphox cells (expressing gp91phox, p22phox, p47phox, and p67phox) were transiently transfected with siRNA p67phox or scrambled siRNA as control. Forty-eight hours after transfection, cell homogenates were prepared and subjected to western blotting using anti-p47phox and anti-p67phox antibodies to verify the effect of siRNA-mediated knockdown (A; bottom) and with different anti-phospho-p47phox antibodies as indicated (A; top). Images shown are representative western blots from 3 independent experiments. (B) The NIH Image J software was used for densitometry analysis and the results expressed as mean ± SEM are shown (n = 3; *P < .05). (C) COS-7 cells were either transiently transfected with gp91phox cDNA in pEF-PGKpac and p22phox/p47phox/p67phox cDNA in pRK5 or transiently transfected with only gp91phox cDNA in pEF-PGKpac and p22phox/p47phox cDNA in pRK5. Twenty-four hours after transfection, the cells were stimulated with vehicle or PMA (200 ng/mL final) for 30 minutes. Cell homogenates were prepared and subjected to western blotting using different anti-phospho-p47phox antibodies, as indicated. (D) Images shown are representative western blots from 3 independent experiments. The NIH Image J software was used for densitometry analysis (n = 3; *P < .05).
p67phox knockdown reduces the phosphorylation of p47phox and its expression increases this process in COSphox cell lines. (A) COSphox cells (expressing gp91phox, p22phox, p47phox, and p67phox) were transiently transfected with siRNA p67phox or scrambled siRNA as control. Forty-eight hours after transfection, cell homogenates were prepared and subjected to western blotting using anti-p47phox and anti-p67phox antibodies to verify the effect of siRNA-mediated knockdown (A; bottom) and with different anti-phospho-p47phox antibodies as indicated (A; top). Images shown are representative western blots from 3 independent experiments. (B) The NIH Image J software was used for densitometry analysis and the results expressed as mean ± SEM are shown (n = 3; *P < .05). (C) COS-7 cells were either transiently transfected with gp91phox cDNA in pEF-PGKpac and p22phox/p47phox/p67phox cDNA in pRK5 or transiently transfected with only gp91phox cDNA in pEF-PGKpac and p22phox/p47phox cDNA in pRK5. Twenty-four hours after transfection, the cells were stimulated with vehicle or PMA (200 ng/mL final) for 30 minutes. Cell homogenates were prepared and subjected to western blotting using different anti-phospho-p47phox antibodies, as indicated. (D) Images shown are representative western blots from 3 independent experiments. The NIH Image J software was used for densitometry analysis (n = 3; *P < .05).
Lack of p67phox does not alter the phosphorylation of proteins unrelated to the NADPH oxidase, and NADPH oxidase-derived ROS are not involved in p47phox phosphorylation in normal neutrophils
To check if the absence of p67phox specifically affects the phosphorylation of p47phox, we analyzed the phosphorylation p38MAPKinase and ERK1/2, which are not part of the NADPH oxidase complex, and showed that their phosphorylation was not affected in neutrophils from p67phox−/− patients with CGD (supplemental Figure 8). Because neutrophils from p67phox−/− patients with CGD do not express the p67phox protein and do not produce ROS, the impairment of p47phox phosphorylation could be due either to the absence of p67phox or the lack of ROS. To investigate whether ROS produced by the NADPH oxidase are involved in p47phox phosphorylation, we isolated neutrophils from healthy donors and treated them with the NADPH oxidase inhibitor DPI, or the superoxide- and H2O2-catabolizing enzymes, SOD and catalase, in the presence or absence of PMA. Results showed that DPI completely inhibited ROS production and that SOD+catalase had a strong antioxidant effect (supplemental Figure 9); however, their addition failed to impair PMA-induced phosphorylation of p47phox (supplemental Figure 9). Similar results were obtained with fMLF as the stimulus (data not shown). Altogether, these results demonstrate that NADPH oxidase-derived ROS are not involved in p47phox phosphorylation and suggest that the decrease in p47phox phosphorylation observed in p67phox−/− cells is not due to the absence of ROS, but rather to the absence of p67phox or associated proteins.
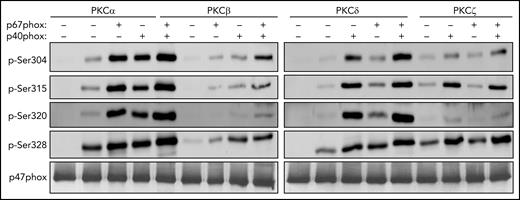
Recombinant p67phox and p40phox enhance the phosphorylation of p47phox by PKC isoforms in vitro
To investigate whether p67phox could enhance the p47phox phosphorylation, we performed in vitro phosphorylation experiments using the PKC isoforms expressed in neutrophils in the presence or absence of p67phox. Results showed that recombinant p47phox alone was weakly phosphorylated by PKC α, β, δ, and ζ; however, the addition of recombinant p67phox enhanced p47phox phosphorylation (Figure 6). Because p40phox was also depleted in p67phox−/− neutrophils, we performed the same in vitro phosphorylation experiments in the absence or presence of p40phox. In the presence of p40phox, p47phox phosphorylation by PKC α, β, δ, and ζ was also mildly enhanced; however, when both p40phox and p67phox were present, p47phox phosphorylation was strongly increased (Figure 6). These results suggest that both p67phox and p40phox are required for optimal p47phox phosphorylation on Ser304, Ser315, Ser320, and Ser328. In addition, we observed that the effect of p67phox on p47phox phosphorylation was dose dependent (Figure 7). Interestingly, heat denaturation of p67phox resulted in reduction of its enhancing effect on PKC-mediated phosphorylation of p47phox, suggesting that the native p67phox is required (Figure 7). Although we only show here the phosphorylation of Ser328, the same results were obtained for the phosphorylation of the other serines (data not shown). In addition, bovine serum albumin, a protein unrelated to the NADPH oxidase and with the same molecular weight as p67phox, had no effect on the phosphorylation of p47phox (supplemental Figure 10).
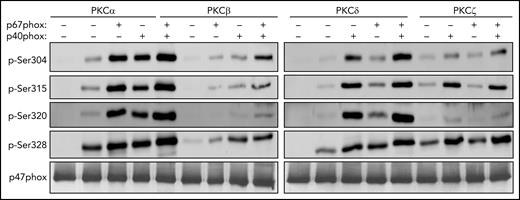
Phosphorylation of recombinant p47phox by purified PKC isoforms in vitro and effect of p67phox and p40phox. Recombinant p47phox protein was incubated without or with p67phox or p40phox or p67phox+p40phox for 10 minutes; PKCα, PKCβ, PKCδ, or PKCζ were added for 5 minutes and the reaction was started by adding ATP, followed by incubation at 30°C for 15 minutes. The reactions were terminated by addition of Laemmli 5× sample buffer and denaturation at 100°C. Proteins were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328 antibodies and anti-p47phox antibody. Experiments were repeated 3 times.
Phosphorylation of recombinant p47phox by purified PKC isoforms in vitro and effect of p67phox and p40phox. Recombinant p47phox protein was incubated without or with p67phox or p40phox or p67phox+p40phox for 10 minutes; PKCα, PKCβ, PKCδ, or PKCζ were added for 5 minutes and the reaction was started by adding ATP, followed by incubation at 30°C for 15 minutes. The reactions were terminated by addition of Laemmli 5× sample buffer and denaturation at 100°C. Proteins were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328 antibodies and anti-p47phox antibody. Experiments were repeated 3 times.
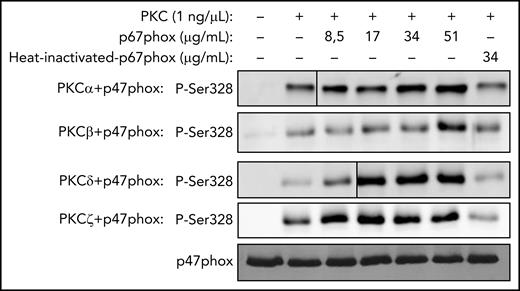
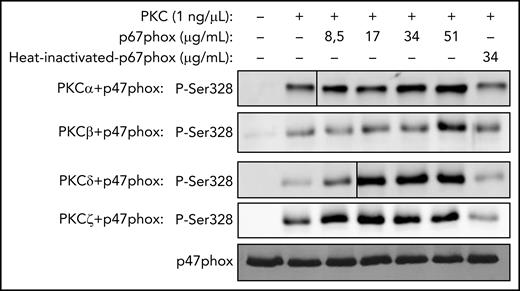
Concentration-dependent effect of p67phox on p47phox phosphorylation by PKC isoforms in vitro and effect of denatured p67phox. Recombinant p47phox protein was incubated without or with different concentrations of p67phox or with heat-denatured p67phox for 10 minutes; PKCα, PKCβ, PKCδ, or PKCζ was added, incubated for 5 minutes, and the reaction was started by adding ATP, followed by incubation at 30°C for 15 minutes. The reactions were terminated by the addition of 5× Laemmli sample buffer and denaturation at 100°C. Proteins were subjected to SDS-PAGE and western blotting using various anti-phospho-p47phox antibodies. Results with anti-phospho-Ser328 antibody are shown. Experiments were repeated 3 times.
Concentration-dependent effect of p67phox on p47phox phosphorylation by PKC isoforms in vitro and effect of denatured p67phox. Recombinant p47phox protein was incubated without or with different concentrations of p67phox or with heat-denatured p67phox for 10 minutes; PKCα, PKCβ, PKCδ, or PKCζ was added, incubated for 5 minutes, and the reaction was started by adding ATP, followed by incubation at 30°C for 15 minutes. The reactions were terminated by the addition of 5× Laemmli sample buffer and denaturation at 100°C. Proteins were subjected to SDS-PAGE and western blotting using various anti-phospho-p47phox antibodies. Results with anti-phospho-Ser328 antibody are shown. Experiments were repeated 3 times.
Discussion
Superoxide production by the phagocyte NADPH oxidase is a tightly regulated process, which requires translocation of the cytosolic subunits to the membrane, leading to enzyme activation.8,10 Among the different processes required for NADPH oxidase activation and ROS production, p47phox phosphorylation is a key step in intact cells.10,11 In the current study, we determined the optimal conditions for the phosphorylation of p47phox in neutrophils stimulated by fMLF and PMA using phosphosite-specific antibodies and investigated the role of p67phox in this process. Stimulation with fMLF and PMA induced the phosphorylation of p47phox on Ser304, Ser315, Ser320, and Ser328, although with different time course. In contrast, Ser345 phosphorylation appeared to be already present at steady state and was only slightly increased by both stimuli. Interestingly, we observed a strong reduction of p47phox phosphorylation in neutrophils from p67phox−/− patients with CGD. Furthermore, in vitro studies showed that the addition of recombinant p67phox alone or to a lesser extent that of p40phox alone, enhanced p47phox phosphorylation on Ser304, Ser315, Ser320, and Ser328, an effect that was further enhanced when both were combined.
It has been known for some time that p47phox is phosphorylated on several serines localized in the carboxy-terminal region.20-22 The initial studies on p47phox phosphorylation in intact neutrophils were performed using [32P]-labeled orthophosphoric acid.20,21 This technique is cumbersome and requires high amounts of radioactive materials, many cells, and a specific antibody capable of immunoprecipitating p47phox. In addition, this technique does not give any information about the individual phosphorylated sites, unless used in tandem with site-directed mutagenesis and 2-dimensional phosphopeptide mapping, 2 very time-consuming techniques. To allow the study of p47phox phosphorylation in cell lysates without the need of radioactivity and immunoprecipitation, we have developed anti-p47phox antibodies highly specific for each serine phosphorylated upon stimulation. These antibodies allow investigation of p47phox phosphorylation by western blotting using few cells, which is an advantage in the case of children with rare diseases.
In this study, we have optimized the experimental protocol to allow for the dynamic capture of p47phox phosphorylation in fMLF- and PMA-stimulated neutrophils. Phosphorylation of Ser304, Ser315, Ser320, and Ser328 from fMLF-stimulated neutrophils reached a peak after only 10 seconds and decreasing over time. This fast and transient phosphorylation of p47phox is consistent with the fast and transient activation of fMLF-induced ROS production by neutrophils. However, the stimulatory effect of PMA on the phosphorylation of Ser304, Ser315, Ser320, and Ser328 occurred more slowly and was sustained for up to 20 minutes (data not shown). Both agonists induced little or no increase in the phosphorylation of Ser345 over that at steady state.
In resting cells, p47phox is in the cytosol either in a free form or in a complex with p67phox.30,31 Indeed, p47phox has 2 SH3 domains and a proline-rich region, whereas p67phox has 2 SH3 domains, with 1 p67phox-SH3 domain interacting with the p47phox proline-rich sequence.32-35 In this study, we had a unique cohort of p67−/−phox patients with CGD, which allowed us to demonstrate for the first time that the absence of p67phox in neutrophils results in a decrease of p47phox phosphorylation. These data were confirmed in patient-derived EBV-transformed lymphocytes and in COSphox cells lacking p67phox, suggesting that p67phox, or an associated protein, is required for optimal p47phox phosphorylation. The decrease of p47phox phosphorylation was less pronounced in COSphox cells than in neutrophils and EBV-L possibly because COSphox cells do not express the same protein kinases and the same PKC isoforms as neutrophils.44,45 Indeed, PKC βII and PKCδ, 2 major PKC isoforms in neutrophils, are not expressed in COSphox cells. Investigation whether the phosphorylation of p47phox by other PKC isoforms or other kinases does not require p67phox would be of interest in the future.
We demonstrated that the effect of p67phox was not observed when cells were deprived of ROS, either by SOD/catalase combination or by pharmacological inhibition of the NADPH oxidase by DPI, suggesting that p67phox is most likely responsible for the increase in p47phox phosphorylation. This critical finding was not reported in previous studies on p67phox−/− patients with CGD using 32P-labeling,46 probably because this technique does not allow to detect each phosphorylated serine but rather reflects the overall phosphorylation. The anti-p47phox antibodies against each phosphorylated site are sensitive and easier to use and may allow to uncover new mechanisms for p47phox phosphorylation. Interestingly, in line with our observation, previous studies reported that phosphorylation of p47phox was also altered in neutrophils from gp91phox-deficient patients with CGD.47,48
We observed that the p40phox subunit was also dramatically depleted in neutrophils from p67phox−/− patients with CGD, as previously described.32,49 In vitro phosphorylation experiments clearly showed that both p67phox and p40phox alone or in combination increased p47phox phosphorylation by PKC isoforms. In addition, we observed that when p67phox was denatured, its effects on p47phox phosphorylation were lost. The decreased phosphorylation of p47phox could be observed in the combined absence of p67phox and p40phox, either in p67phox−/− neutrophils or in p67phox siRNA-treated COSphox cells that do not express p40phox. These results suggest that a p47phox/p67phox/p40phox interaction may favor p47phox phosphorylation. Alternatively, a protein associated with p67phox and p47phox may be involved; thus, the mechanism of this impairment should be studied in more detail in future studies. In line with our observations, p67phox and p40phox were shown to enhance NADPH oxidase activation in a cell-free activation system.50-54
In summary, this study characterized the phosphorylation of p47phox in fMLF- and PMA-stimulated neutrophils on Ser304, Ser315, Ser320, and Ser328 by using anti-phospho-p47phox antibodies and showed that p67phox and p40phox are novel regulators of p47phox-phosphorylation in human neutrophils. This study and the developed tools may facilitate investigations on the role of phosphorylation in NADPH oxidase activation.
Acknowledgments
The authors thank Martine Torres for her editorial assistance.
This work was supported by grants from INSERM, CNRS, University de Paris, the Labex Inflammex, VLM (vaincre la mucoviscidose), the Algerian Ministry of Research (Fellowship for S.A.B.), and the Shenzhen Bay Laboratory Open Project SZBL2020090501011 (R.D.Y.).
Authorship
Contribution: S.A.B. designed and conducted the experiments and wrote the manuscript; V.M., M.H.-N., C.P., Y.L., S.L., and T.B. conducted experiments and wrote the manuscript; M.-A.G.-P. wrote the manuscript; R.D.Y. and P.M.-C.D. designed experiments and wrote the manuscript; and J.E.-B. designed and supervised experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Jamel El-Benna, Faculté de Médecine Xavier Bichat, Centre de Recherche sur l'Inflammation, INSERM-U1149, 16 rue Henri Huchard, Paris F-75018, France; e-mail: jamel.elbenna@inserm.fr.
REFERENCES
Author notes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
P.M.-C.D. and J.E.-B. contributed equally to this study.

![Kinetics and concentration-dependent effect of fMLF on p47phox phosphorylation and ROS production in human neutrophils. (A) Human neutrophils (15 × 106/400 µL) were incubated with fMLF (1 µM) for different times and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328, and anti-phospho-Ser-345 antibodies and anti-p47phox antibody. (B) Neutrophils (0.5 × 106/500 µL) were incubated in HBSS for 5 minutes in the presence of 10 µM luminol at 37°C and stimulated with fMLF (1 µM); ROS production was measured by luminol-amplified chemiluminescence over time. (C) Human neutrophils (15 × 106/400 µL) were incubated with different concentrations of fMLF for 10 seconds and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using the same antibodies as in panel A. (D) ROS production of neutrophils (0.5 × 106/500 µL HBSS) was measured as in panel B. Experiments were repeated 3 times (n = 3, mean ± standard error of the mean [SEM]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/139/16/10.1182_blood.2021011134/5/m_bloodbld2021011134f1.jpeg?Expires=1765954835&Signature=AkXotV~JBzPYKPbwWFxtOiSDk6hx6GvtaOGBusn1qqjyw7CnDBCTQ8lw99XmBUk04mVMuyJOM3ETkLBjQZ34YqWfyQXbYBDpB7sIpPrITmK4jDudrG1Vz3y2ylUTfdeK8KlsuDFPiM7lQXW-7tndPyepkc7u2nJX0lridwmxbbasVEb9tuou65dJey8jXgQTpiXL1OHCebxUsW1aOXGDgbfIXIqQnbGhglABHAMWx782I17dxVa9JGjAEtWIkcYyoF1tXU09xLSrURje37eGt4vRGoOqbo8a6oIw6yVWHVokuEZyFngQS0EeZrZishw4VV56IboSI2YdtTo1GiYgCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Kinetics and concentration-dependent effect of fMLF on p47phox phosphorylation and ROS production in human neutrophils. (A) Human neutrophils (15 × 106/400 µL) were incubated with fMLF (1 µM) for different times and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using different anti-phospho-p47phox antibodies: anti-phospho-Ser304, anti-phospho-Ser315, anti-phospho-Ser320, anti-phospho-Ser328, and anti-phospho-Ser-345 antibodies and anti-p47phox antibody. (B) Neutrophils (0.5 × 106/500 µL) were incubated in HBSS for 5 minutes in the presence of 10 µM luminol at 37°C and stimulated with fMLF (1 µM); ROS production was measured by luminol-amplified chemiluminescence over time. (C) Human neutrophils (15 × 106/400 µL) were incubated with different concentrations of fMLF for 10 seconds and the reaction was stopped by denaturation with 100 µL hot Laemmli sample buffer (5×). Homogenates were subjected to SDS-PAGE and western blotting using the same antibodies as in panel A. (D) ROS production of neutrophils (0.5 × 106/500 µL HBSS) was measured as in panel B. Experiments were repeated 3 times (n = 3, mean ± standard error of the mean [SEM]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/139/16/10.1182_blood.2021011134/5/m_bloodbld2021011134f1.jpeg?Expires=1766021692&Signature=UFcJueaJbxZXAz3J~JBF72rQyt61em20KqSfOF4BclPPVJ8El-72sZBECiBRbjf2mXIpoRG0k9iJlOSt0vqgT0bD3FgaMKdja7Smxx5Hzy0AqE-JYsOOwi0km7jQZLmoBY~rgwjCUjc81AiweyDsO4b2KErEDbNflnNnnVVhhQ8syUlCaW0vGD948~0Yi4W2J66ZFkxoNYgXRP4ZxtsEcxIxjr~uwC9An~2KQA7AYVrbIYhRdNUXFcF~Z3kGMbJp730J3UmdaS1MHpb4D7qiUGjKPuhTbtkGF4nOXYipsRTWj6prTRkng0tt5pMULTlWvPveeC25IzKZCItAS4VajA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)