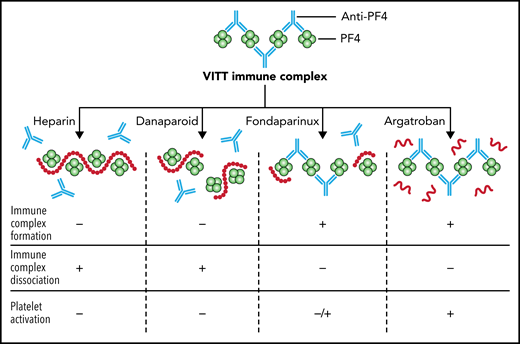
In this issue of Blood, Singh et al1 examined the interaction of heparin, danaparoid, fondaparinux, and argatroban with antibodies against platelet factor 4 (PF4) isolated from patients with vaccine-induced thrombotic thrombocytopenia (VITT). The goal was to determine which anticoagulant(s) could inhibit platelet activation and thrombosis in vitro, and provide biochemical data to inform treatment options for what is now considered one of the most potent prothrombotic conditions ever observed.
VITT was a mysterious thrombotic disorder that was observed during the mass rollout of the adenoviral vector vaccine ChAdOx1 nCoV-19 (AstraZeneca) first in Europe and then in other parts of the world 1 year into the COVID-19 pandemic. The syndrome seemed to affect predominantly young healthy women, but this was more likely a reflection of the population of health care workers who were first in line for vaccination. Although the absolute number of affected individuals was small, the presentation was dramatic and outcomes were devastating. In an attempt to explain the acute onset of thrombosis and thrombocytopenia when it initially appeared, clinicians tested broadly for possible causes, including the antiphospholipid antibody syndrome, thrombotic thrombocytopenic purpura, and heparin-induced thrombocytopenia (HIT), even though patients had not been exposed to heparin. Unexpectedly, all patients had very high levels of anti-PF4 antibodies and demonstrated positive results in platelet activation assays with the addition of exogenous PF4. Given the similarities with HIT, treatment recommendations for VITT were rapidly adopted, including the use of nonheparin anticoagulants and adjunct intravenous immunoglobulin.2
Although this treatment strategy seemed to work, nonheparin anticoagulants have several disadvantages. For one, they can be difficult to reverse, which is especially concerning because of the high frequency of cerebral venous sinus thrombosis and associated intracerebral bleeding. In addition, nonheparin anticoagulants are less accessible in low- and middle-income countries where VITT is an ongoing concern. Indeed, adenoviral vector vaccines have many attractive features, including room temperature storage, long shelf life, and relatively low cost, making them an appealing choice for vaccines for COVID-19 and other infectious diseases in many parts of the world. Thus, investigating the biochemical and clinical effects of heparin and nonheparin alternatives as potential treatments for VITT is an important area of research.
In a series of functional and serological experiments, Singh and colleagues showed that heparin and danaparoid inhibited thrombus formation ex vivo, inhibited the formation of PF4/anti-PF4 immune complexes, caused the dissociation of preformed immune complexes, and inhibited platelet activation (see figure). Fondaparinux did not inhibit the formation of new immune complex or cause the disruption of preformed immune complexes, but it modestly inhibited platelet activation. Argatroban had no effect on immune complexes or platelet activation. The ability of heparin to dissociate PF4/anti-PF4 complexes follows recent molecular studies showing that VITT antibodies bind to precisely the same amino acids on PF4 as heparin.3 Danaparoid, a low-molecular-weight heparinoid that consists predominantly of dermatan sulfate and low-sulfated heparan sulfate, presumably also binds to positively charged PF4 and thus can displace VITT antibodies in a similar manner, because it has been shown to inhibit the formation of PF4-heparin complexes.4
The effect of unfractionated heparin, danaparoid, fondaparinux, and argatroban on PF4/anti-PF4 immune complexes from patients with VITT. Heparin and danaparoid inhibit the formation of PF4/ anti-PF4 immune complexes (IC), cause the dissociation of pre-formed ICs, and inhibit platelet activation. Fondaparinux does not inhibit IC formation and does not cause the dissociation of pre-formed ICs, but modestly inhibits platelet activation. Argatroban has no effect on ICs or platelet activation. Professional illustration by Patrick Lane, ScEYEnce Studios.
The effect of unfractionated heparin, danaparoid, fondaparinux, and argatroban on PF4/anti-PF4 immune complexes from patients with VITT. Heparin and danaparoid inhibit the formation of PF4/ anti-PF4 immune complexes (IC), cause the dissociation of pre-formed ICs, and inhibit platelet activation. Fondaparinux does not inhibit IC formation and does not cause the dissociation of pre-formed ICs, but modestly inhibits platelet activation. Argatroban has no effect on ICs or platelet activation. Professional illustration by Patrick Lane, ScEYEnce Studios.
These basic mechanistic data provide important biological information about VITT-induced thrombosis; however, the safety of heparin as a treatment for VITT remains uncertain. Evidence in favor of its use include in vitro studies like the one by Singh and colleagues showing that platelet activation induced by VITT sera can be inhibited by the addition of pharmacological concentrations of heparin,5 as opposed to HIT where the addition of pharmacological heparin enhances platelet activation in vitro. In addition, there have been several reports of patients with VITT who were treated with unfractionated heparin without evidence of worsening outcomes.6 On the other hand, recent data have shown that some VITT antibodies can bind to sites on PF4 that are distinct from the heparin binding site, as described in a report of VITT following the Ad26.COV2.S (Johnson & Johnson/Janssen) vaccine.7 Thus, it is possible that heparin can enhance platelet activation in some VITT patients, similar to HIT. Furthermore, in a large clinical study of patients with VITT (n = 220), mortality was higher among patients who received unfractionated heparin compared with nonheparin alternatives (10/50 [20%] vs 27/170 [16%]).8
Despite the rapid decline in the incidence of VITT and the discontinuation of adenoviral vector vaccines for COVID-19 in many countries, there is an ongoing need for further clinical and basic research in this highly prothrombotic disorder. Adenoviral vector vaccines will continue to be used for COVID-19 in many countries; thus, there is a global responsibility to optimize their safety. In addition, some patients with VITT continue to show serological evidence of anti-PF4 antibodies and clinical morbidities even 1 year later (“long VITT”9), and optimizing their treatment is a priority. Finally, understanding the mechanisms by which anti-PF4 antibodies alone can cause such severe thrombosis provides a unique opportunity to advance our understanding of the intersection between immunity and thrombosis.10
Conflict-of-interest disclosure: The author declares no competing financial interests.