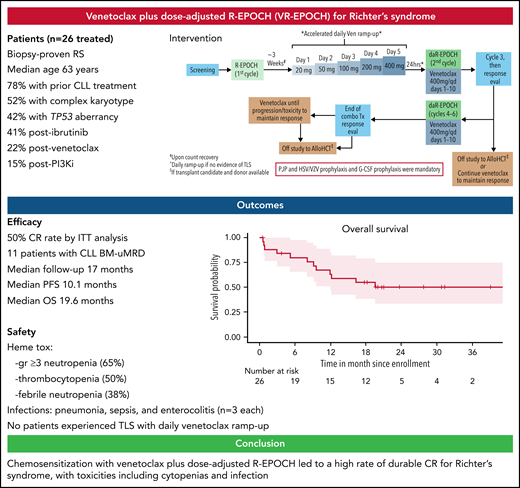
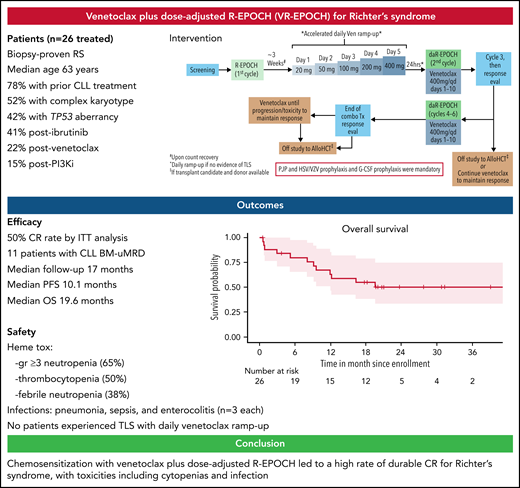
Key Points
VR-EPOCH led to a high rate of durable CR for patients with RS.
Toxicities included cytopenias and infection, but tumor lysis syndrome was not observed with accelerated daily venetoclax ramp-up.
Abstract
Richter syndrome (RS) of chronic lymphocytic leukemia (CLL) is typically chemoresistant, with a poor prognosis. We hypothesized that the oral Bcl-2 inhibitor venetoclax could sensitize RS to chemoimmunotherapy and improve outcomes. We conducted a single-arm, investigator-sponsored, phase 2 trial of venetoclax plus dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (VR-EPOCH) to determine the rate of complete response (CR). Patients received R-EPOCH for 1 cycle, then after count recovery, accelerated daily venetoclax ramp-up to 400 mg, then VR-EPOCH for up to 5 more 21-day cycles. Responders received venetoclax maintenance or cellular therapy off-study. Twenty-six patients were treated, and 13 of 26 (50%) achieved CR, with 11 achieving undetectable bone marrow minimal residual disease for CLL. Three additional patients achieved partial response (overall response rate, 62%). Median progression-free survival was 10.1 months, and median overall survival was 19.6 months. Hematologic toxicity included grade ≥3 neutropenia (65%) and thrombocytopenia (50%), with febrile neutropenia in 38%. No patients experienced tumor lysis syndrome with daily venetoclax ramp-up. VR-EPOCH is active in RS, with deeper, more durable responses than historical regimens. Toxicities from intensive chemoimmunotherapy and venetoclax were observed. Our data suggest that studies comparing venetoclax with chemoimmunotherapy to chemoimmunotherapy alone are warranted. This trial was registered at www.clinicaltrials.gov as #NCT03054896.
Introduction
Despite improved outcomes for patients with chronic lymphocytic leukemia (CLL), transformation into diffuse large B-cell lymphoma, known as Richter syndrome (RS), remains a major unmet need. Patients with RS have a median overall survival (OS) of only 3 to 6 months, with no established standard-of-care treatment.1,2 Anthracycline-based chemoimmunotherapy regimens such as rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R-EPOCH) are often used to treat RS, but rates of complete remission (CR) are only ∼20%, and the progression-free survival (PFS) and OS with R-EPOCH are 3.5 and 5.9 months, respectively.3 The oral BCL-2 inhibitor venetoclax, which is approved to treat CLL,4 has single-agent activity in RS, with 3 of 7 patients responding in a phase 1 study.5 We hypothesized that venetoclax could achieve synergy with chemoimmunotherapy, thereby providing deep and durable response. We therefore initiated this phase 2 study of venetoclax plus dose-adjusted R-EPOCH (VR-EPOCH) for patients with RS.
Study design
Patients were included if they had RS, defined as a confirmed diagnosis of CLL by standard criteria6 with biopsy-proven diffuse large B-cell lymphoma, had adequate hematologic parameters, performance status, and organ function. Full eligibility criteria are provided in the supplemental data on the Blood Web site. R-EPOCH was given for 1 cycle, followed after count recovery by accelerated inpatient venetoclax ramp-up (20 mg initial dose with escalating dose on a daily basis to 50 mg, 100 mg, 200 mg, and finally 400 mg daily) with close monitoring for tumor lysis syndrome. Venetoclax dose ramp-up was started at the end of cycle 1 (days 22-27), with day 1 of cycle 2 starting the day after 400 mg was reached. Patients then received up to 5 cycles of VR-EPOCH in 21-day intervals, with venetoclax dosed at 400 mg daily on days 1 through 10 of each cycle and standard R-EPOCH dose adjustment.7 All cycles of R-EPOCH were given in an inpatient setting. Responders could come off study to receive cellular therapy at any time (supplemental Figure 1) or could receive continuous daily venetoclax 400 mg maintenance. Granulocyte colony-stimulating factor and prophylaxis against Pneumocystis jiroveci pneumonia and herpes viruses were mandatory. Response evaluation for RS was performed by Lugano criteria8 including positron emission tomography/computed tomography at regular intervals. Minimal residual disease (MRD) assessment for CLL in the bone marrow was performed by flow cytometry (sensitivity of ≥10-4) in patients in CR. Toxicities were assessed by Common Terminology for Adverse Events, version 4. All patients who received any study treatment were included in the efficacy and safety analyses, which are reported descriptively, with a data cutoff date of June 1, 2021. The primary endpoint was the rate of CR in an efficacy evaluable population defined as patients who started VR-EPOCH combination therapy. Survival curves were estimated using the Kaplan-Meier method. The institutional review board at each site approved the protocol, and all participants provided written informed consent.
Results and discussion
Twenty-seven patients were enrolled at 3 sites. One patient never received any study therapy because of an infection. Baseline patient characteristics are summarized in supplemental Table 1. The median age at enrollment was 63 years (range, 49-77), with 42% of patients having CLL with TP53 aberrant disease and 52% with complex karyotype. In the 11 patients in whom clonal relatedness of RS to prior CLL could be determined, 9 (82%) were clonally related. Patients had a median of 1 prior therapy for CLL (range, 0-7), including 14 patients with prior novel agent therapy (52%). Six patients (22%) were previously untreated for CLL, and 2 patients had prior treatment of RS (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone; [R-CHOP] blinatumomab).
Twenty-six patients started cycle 1 of R-EPOCH, with 20 patients starting cycle 2 (VR-EPOCH). Efficacy outcomes are summarized in Table 1. Thirteen of 20 (65%; 95% confidence interval [CI], 0.41-0.85]) who received VR-EPOCH achieved CR (primary endpoint). The best overall response rate, including 3 additional evaluable patients with partial response, was 16/20 (80%). Among all patients who received any study therapy, the CR rate was 13/26 (50%) and overall response rate 16/26 (62%). Five of the 9 (56%) evaluable patients who previously progressed on ibrutinib, and both patients who progressed to RS while on venetoclax, achieved CR. Of the known clonally related patients, 7 of the 9 achieved response, including 5 with CR. Eleven of the 12 patients who achieved CR and had a bone marrow biopsy had undetectable MRD for CLL. Nine of 18 (50%) cellular therapy candidates underwent subsequent allogeneic transplantation (n = 8) or chimeric antigen receptor T-cell therapy (n = 1). Eleven patients received subsequent venetoclax maintenance.
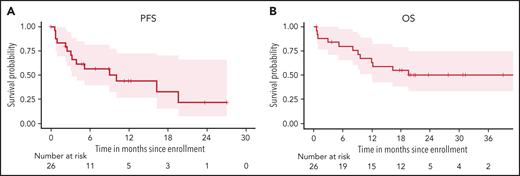
With a median follow-up of 17 months (range, 0.6-42), the median PFS is 10.1 months (95% CI, 3.2-NA) (Figure 1A), and median OS is 19.6 months (95% CI, 12-NA) (Figure 1B). PFS in patients with complex karyotype was significantly shorter than those without (median 3.1 vs 19.6 months, respectively; P = .015), but there was no difference in OS (P = .23) (supplemental Figure 2A,B). There was no difference in PFS or OS based on TP53 status (supplemental Figure 2C,D). The median number of cycles of VR-EPOCH received was 4 (range, 0-6). Six patients required dose de-escalation of R-EPOCH because of hematologic toxicities, and 1 patient escalated R-EPOCH dosing. Four patients had venetoclax dose reduction because of persistent neutropenia or need for concomitant medication. Twelve patients have died: 8 from disease progression, 2 from postallogeneic transplantation nonrelapse mortality, and 1 each during cycle 1 before venetoclax from sepsis and sudden death (presumed to be cardiopulmonary).
Survival of patients with Richter syndrome with VR-EPOCH. (A) PFS in all 26 patients who received any study therapy. (B) OS in all 26 patients who received any study therapy.
Survival of patients with Richter syndrome with VR-EPOCH. (A) PFS in all 26 patients who received any study therapy. (B) OS in all 26 patients who received any study therapy.
Key adverse events are summarized in Table 1. The most common toxicities were hematologic: neutropenia (65% ≥grade 3, including 58% grade 4), anemia (62%, all grade 3), and thrombocytopenia (50% ≥grade 3, including 42% grade 4). Febrile neutropenia occurred in 38% of patients (26% ≥grade 3, including 12% grade 4). Notable ≥grade 3 infections included sepsis during cycle 1 of R-EPOCH (n = 3, which in 1 case was fatal), pneumonia (n = 3), enterocolitis (n = 3), and sinusitis (n = 2). No opportunistic infections were observed. Notably, we safely ramped up all 21 patients who received venetoclax to 400 mg in 5 days without tumor lysis syndrome. These are the first prospective clinical trial data of daily venetoclax ramp-up in patients with B-cell malignancies, a strategy now also being explored in CLL (NCT04843904).
Our results with VR-EPOCH demonstrate, to our knowledge, the highest CR rate (50%) of any published study in RS, with a promising median OS of 19.6 months in patients typical of a modern RS population. Of note, one-half of the patients deemed at study entry to be candidates for cellular therapies were able to undergo allogeneic transplantation (n = 8) or chimeric antigen receptor T-cell therapy (n = 1), suggesting that VR-EPOCH as a bridge to cellular therapies may be a viable curative strategy for some patients. VR-EPOCH was active both in patients with clonally related and unrelated RS, and similarly in patients with and without TP53 mutation. Complex karyotype was the one predictor of shorter PFS in our study, suggesting a particular need to explore alternative therapeutic options for this group. Although our multicenter study is relatively small, it is one of the largest prospective clinical trials focused specifically on RS. To further explore the promise of this approach in RS, we recently opened an expansion cohort to explore venetoclax plus rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone (VR-CHOP).
In summary, VR-EPOCH led, to our knowledge, to the highest CR rate and longest median OS reported in a study of patients with RS. Given that no effective standard therapy exists for RS, our data support consideration of using VR-EPOCH in clinical practice in appropriate patients with close monitoring. An eventual future comparative study should explore venetoclax + chemoimmunotherapy vs chemoimmunotherapy alone in RS.
Acknowledgments
The authors thank the patients and their families and friends who supported them in participating in this trial and acknowledge the study research nurses, research coordinators, advanced practice practitioners, and site staff for their support of the trial.
This study was funded by Genentech/AbbVie, with Richter syndrome whole exome sequencing for clonality analysis based upon sequencing data supported by the Broad Institute/IBM Cancer Resistance Research Project. M.S.D. and K.A.R. are both Scholars in Clinical Research from the Leukemia & Lymphoma Society.
Authorship
Contribution: M.S.D., K.A.R., P.A.T., and J.R.B. contributed to the overall design, performed research, collected, analyzed, and interpreted data, and prepared and wrote the manuscript; T.Z.L., E.M.P., and C.J.W. performed research; M.S.D., S.T., Z.W., and S.P. analyzed the data and performed statistical analyses; M.S.D., K.A.R., S.K.R., J.M., U.I., C.A.J., D.C.F., P.A.T., and J.R.B. provided clinical care and collected data; M.S.D. supervised the study and served as the Principal Investigator and Sponsor-Investigator; and all authors critically reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.S.D. reports receiving grant support, paid to his institution, and consulting fees from Ascentage Pharma, Astra-Zeneca, Genentech, MEI Pharma, Pharmacyclics, TG Therapeutics, and Verastem; grant support, paid to his institution from BMS and Surface Oncology; and consulting fees from AbbVie, Adaptive Biotechnologies, Aptitude Health, BeiGene, Celgene, Eli Lilly, Janssen, Merck, Research to Practice, and Takeda. K.A.R. reports receiving grant support, paid to her institution, and consulting fees from AbbVie and Genentech; grant support, paid to her institution from Janssen and Novartis; and consulting fees from Acerta Pharma, AstraZeneca, Innate Pharma, and Pharmacyclics. C.J.W. is an equity holder of BioNTech and reports receiving grant support, paid to her institution, from Pharmacyclics. P.A.T. reports receiving grant support, paid to his institution, from AbbVie, Adaptive Biotechnologies, Amgen, Genentech, and Pharmacyclics, and consulting fees from Abbvie, Adaptive Biotechnologies and Janssen. J.R.B. reports receiving consulting fees from AbbVie, Acerta, Astra-Zeneca, BeiGene, Catapult, Dynamo Therapeutics, Eli Lilly, Genentech/Roche, Gilead, Juno/Celgene/Bristol Myers Squibb, Kite, Loxo, MEI Pharma, Nextcea, Novartis, Octapharma, Pfizer, Pharmacyclics, Rigel, Sunesis, TG Therapeutics, and Verastem; honoraria from Janssen and Teva; institutional research funding from Gilead, Loxo, Sun, TG Therapeutics, and Verastem; and has served on data safety monitoring committees for Morphosys and Invectys. The remaining authors declare no competing financial interests.
The current affiliation for T.Z.L. is Casma Therapeutics, Cambridge, MA.
Correspondence: Matthew S. Davids, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: matthew_davids@dfci.harvard.edu.
Presented in part as oral abstracts at t he 2019 International Conference on Malignant Lymphoma in Lugano, Switzerland, 19-23 June 2019, and the 2020 American Society of Clinical Oncology Virtual Meeting, 29 May-2 June 2020.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
M.S.D. and K.A.R. are joint first authors who contributed equally to this study.
P.A.T. and J.R.B. are joint senior authors who contributed equally to this study.