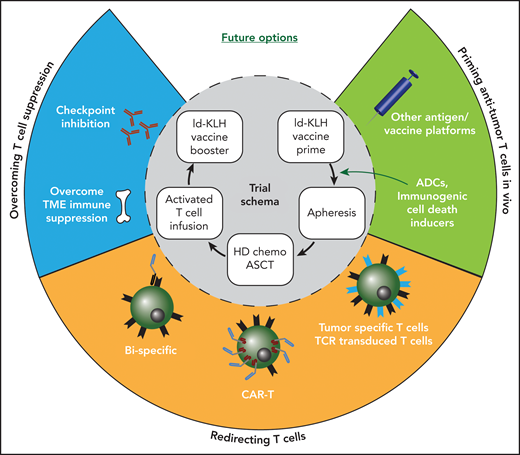
In this issue of Blood, Qazilbash et al1 demonstrate the feasibility of delivering costimulated T cells from patients with idiotype vaccine-primed myeloma (MM) in the peritransplant setting, followed by additional booster idiotype vaccination.
Immune cells, and particularly T cells, are a potent weapon against MM.2 Antigen specificity and immunologic memory, with its potential to mediate long-term protection, are 2 critical attributes of T cells, which make them important targets for immunotherapy. The capacity of T cells to mediate antimyeloma activity has now been well established with the clinical success of T-cell redirection strategies such as chimeric-antigen receptor (CAR) T cells and T-cell targeting bispecific antibodies.2 In both approaches, relative tumor-selectivity is achieved via tumor-targeting single chain variable fragments, but the capacity for durable MM immune control or cure remains elusive. Vaccines are the most common approach to engage antigen-specific immunity; vaccines against pathogens represent one of the biggest triumphs of modern medicine and can lead to lifelong protection against pathogens.3 In contrast, therapeutic vaccines against cancer have achieved limited clinical efficacy. A critical component of vaccine design is the choice of target antigen(s). Idiotypic determinants on clonal immunoglobulin expressed by tumor cells represent a unique tumor-specific antigen in B/plasma cell malignancies and have been the target of several immune therapeutic approaches.4,5 Adoptive T-cell therapeutic strategies recently investigated in myeloma have included blood/marrow T cells following ex vivo costimulation and those engineered to express tumor-specific T-cell receptors (TCRs) or CAR T cells.2 Prior studies by the Penn group have demonstrated the capacity of costimulated T cells to enhance pathogen or tumor antigen–specific immunity derived from prior vaccines, although the ex vivo stimulation was not antigen specific.6,7 Qazilbash et al combine 2 approaches pioneered by this team using an idiotype vaccine to prime T cells from patients with MM in vivo and then deliver the ex vivo costimulated T cells after high-dose chemotherapy and stem cell transplant, followed by additional booster idiotype vaccination (see figure). The strategy was safe, feasible and was associated with the induction of idiotype-specific T-cell responses. However, whether high-affinity T cells with antitumor function were elicited in the bone marrow with this approach remains to be shown. Clinical outcomes in both arms (receiving experimental idiotype-keyhole limpet hemocyanin [KLH] or KLH-alone control arm) were comparable. Other limitations of the study, including the small sample size and inability to achieve the planned target, may have also contributed to inability to discern a clinical benefit.
Combining immunotherapy approaches in myeloma. Inner circle depicts the idiotype (Id) vaccine trial schema used by Qazilbash et al. Opportunities to combine other immunotherapy approaches are depicted in the outer circle. ADCs, antibody-drug conjugates; ASCT, autologous stem cell transplant; HD, high dose; TME, tumor microenvironment.
Combining immunotherapy approaches in myeloma. Inner circle depicts the idiotype (Id) vaccine trial schema used by Qazilbash et al. Opportunities to combine other immunotherapy approaches are depicted in the outer circle. ADCs, antibody-drug conjugates; ASCT, autologous stem cell transplant; HD, high dose; TME, tumor microenvironment.
What then can we learn from this elegant study and where do we go from here (see figure)? As this approach did not address immune suppressive elements in MM, combination approaches with immune checkpoint inhibitors, immune modulatory drugs, or targeting other immune suppressive factors in the MM tumor bed could be an obvious next step.2 In view of the recent clinical success of bispecifics and CAR T cells in MM, an important next step is to better understand the mechanisms that underlie response, resistance, and, most importantly, durability of response to these T-cell redirection therapies in patients with MM. To combine these strategies with vaccines or other aspects of endogenous or antigen-specific immunity, a better understanding is needed for the biology of endogenous antigen-specific T cells, including against targets other than the idiotype, for example, wherein the detection of immune response has been linked to clinical outcome.8 CAR T cell–based approaches wherein specific endogenous TCRs are retained may permit novel combination approaches, including using vaccines. Future studies that integrate immunotherapy into MM management will also need to consider interpatient diversity in terms of T-cell states, as is being shown in current studies.9 Progressive T-cell exhaustion over time, particularly in terms of attrition of stem-like memory T cells in MM, may elicit the need to consider immune therapies earlier in disease course (ie, with fewer prior lines of therapy). Deeper understanding of immunologic health in our patients is critical for their care, as illustrated by the COVID-19 pandemic.3 Although much of the current focus on immune approaches in MM relates to therapy, a bigger impact of immune approaches in MM may be in the setting of prevention, as illustrated recently by the remarkable success of immune-modulatory therapies to prevent clinical MM.10 We are currently in the midst of an immune revolution in MM therapeutics, and we eagerly await unlocking the full potential of the immune system to cure and prevent this malignancy. For that, we need to learn how to optimally combine different immune approaches and learn to tango.
Conflict-of-interest disclosure: The authors declare no competing financial interests.