In this issue of Blood, Chen et al1 demonstrate that Grab, a Rab guanine exchange factor for Rab8, regulates transferrin (Tf)‐bound iron uptake in mouse fetal liver erythroblasts, zebrafish embryos, and human leukemic cell lines. Prior studies found that polymorphisms in GRAB and its target, RAB8, affect the mean corpuscular hemoglobinization of red blood cells. Together these findings suggest that Grab is another potential target to regulate Tf-mediated iron uptake into erythropoietic cells.
Approximately 70% of all the iron in the body is incorporated into hemoglobin in red blood cells. Tf-mediated iron uptake into erythroblasts is regulated by extent of iron-bound Tf, the number of Tf receptors (Tfrc), and the rates of endocytosis and exocytosis of Tfrc. The mechanisms by which the Tfrc is recycled to the plasma membrane are the subject of this paper. The authors chose the hbd mice because these mice have a spontaneous mutation resulting in a hypochromic‐microcytic anemia.2 A previously published paper indicated that the reticulocytes of hbd mice showed defects in Tf uptake, and in Tfrc recycling.3 Mapping and sequencing of the mutation identified a 24-amino-acid deletion in Sec15l1 (Sec15) as the culprit causing the anemia.4,5 Sec15 is a subunit of the exocyst, an octameric protein complex that facilitates the tethering of recycling vesicles to the plasma membrane.6 These studies indicate that the exocyst and proteins that interacted with the exocyst were candidate of interest.
Experiments in the present paper provide mechanistic insights as to how a guanine nucleotide exchange factor (GEF), Grab, interacts with the exocyst and affects recycling of Tfrc containing vesicles. Prior studies indicated that Grab was upregulated during erythroid differentiation.7 The authors examine the consequences of erythropoietic cells deficient in Grab on the recycling of Tfrc containing vesicles to the plasma membrane. They use embryonic zebrafish to study the role of Tf‐mediated uptake in the regulation of erythropoiesis. They also use erythroblasts from fetal mouse liver cells and 2 cell lines, MEL and K562, to study differentiation of erythropoietic cells. Immunoblots of Grab and Tfrc demonstrate upregulation of both proteins in erythropoietin‐induced differentiation of erythroid progenitors of fetal mouse liver cells. In addition, Na butyrate–induced differentiation of K562 cells and dimethyl sulfoxide–induced differentiation of MEL cells recapitulate the upregulation of Grab. Other experiments show that Grab is predominantly expressed in the bone marrow. Knockdown of Grab in zebrafish embryos, MEL cells, and K562 cells decreases the hemoglobin and heme levels in these models of erythroid development and differentiation. The heme effect in Grab‐deficient cells is directly related to decreased Tf‐mediated iron accumulation in erythroblasts. The decrease in hemoglobin and heme levels is iron mediated because they can be overridden by supplying cells with iron, independently of Tf‐mediated delivery. These experiments indicate that Grab plays a key role in regulating the amount of iron provided to cells via Tfrc trafficking and downregulation of Tfrc levels in cells.
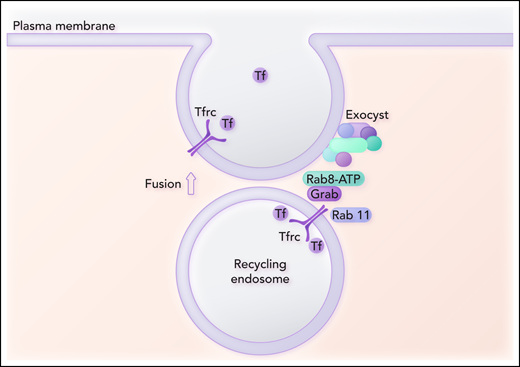
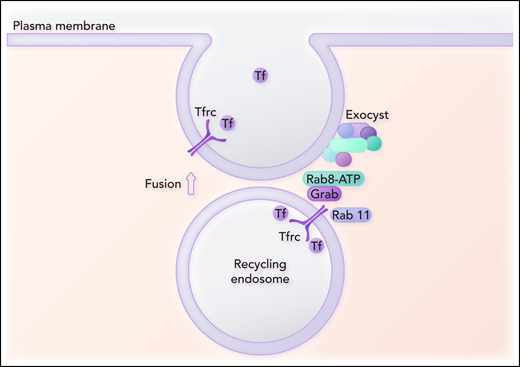
Grab is a GEF, and GEFs activate Rabs. The authors then explore the role that Grab plays in vesicle recycling to the cell surface by identifying key proteins involved in the interaction of the recycling vesicle with the exocyst. Coimmunoprecipitation of a Flag‐tagged Grab followed by mass spectroscopy analysis indicates that the only Rab protein detected is Rab8. Knockout of Rab8 in MEL cells results in decreased Tfrc levels and heme production to a comparable extent as in Grab knockout cells. The authors postulate that Rab8, activated by Grab, recruits the exocyst to the recycling endosome (see figure). They propose that if Tfrc fails to recycle to the plasma membrane, the vesicles are diverted to lysosomes to account for the downregulation of Tfrc at the protein level.
Grab serves as a guanine exchange factor for the activation of Rab8. Grab also interacts with Tfrc and Rab11. Activated Rab8 is postulated to bind to the Sec15 subunit of the exocyst complex and thus recruits the exocyst complex to the recycling endosome. Another subunit of the exocyst complex interacts with the plasma membrane and is involved in the assembly of the fusion machinery. Fusion of the recycling endosome with the plasma membrane promotes exocytosis of Tf and completes the recycling Tfrc to the plasma membrane. Professional illustration by Somersault18:24.
Grab serves as a guanine exchange factor for the activation of Rab8. Grab also interacts with Tfrc and Rab11. Activated Rab8 is postulated to bind to the Sec15 subunit of the exocyst complex and thus recruits the exocyst complex to the recycling endosome. Another subunit of the exocyst complex interacts with the plasma membrane and is involved in the assembly of the fusion machinery. Fusion of the recycling endosome with the plasma membrane promotes exocytosis of Tf and completes the recycling Tfrc to the plasma membrane. Professional illustration by Somersault18:24.
As with any new data, many other questions arise from the studies. Is there heterogeneity or cell-type specificity in recycling endosomes? The authors show a difference in behavior of 2 recycling endocytic markers, Rab11 and Rab35, in Grab knockout cells. Does Tfrc associate with Grab? Immunoblots of coprecipitation studies indicate that Tfrc coisolates with Grab, but mass spectrometry analysis of Grab complexes shows no detectable interaction with Tfrc. In the absence of transport to the periphery, how is Tfrc decreased? Is it delivered to a degradation pathway by fusion with lysosomes or packaged into exosomes for release from cells? These issues need to be explored in future experiments.
The results of this study suggest that Grab expression in erythropoiesis may be a new avenue to control iron levels in red blood cells. Prior papers indicated that polymorphisms in GRAB and its target RAB8 affect the mean corpuscular hemoglobinization of red blood cells.8,9 Because the authors demonstrated that Grab is primarily expressed in the bone marrow, it may be a specific target for controlling iron‐uptake in erythropoietic cells.
Conflict-of-interest disclosure: The authors declare no competing financial interests.